Jul. 28, 2017 Research Highlight Chemistry
Scientists map nickel-based catalyst’s electrons and reveal acid–base action
Crystallographers and synthetic chemists collaborate to reveal how a common nickel catalyst acts to assemble molecules
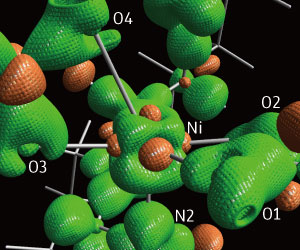 Figure 1: X-ray diffraction data reveals the electronic structure at the heart of a nickel catalyst. Reproduced from Ref. 1 and licensed under CC BY 4.0. © 2017 Y. Sohtome et al.
Figure 1: X-ray diffraction data reveals the electronic structure at the heart of a nickel catalyst. Reproduced from Ref. 1 and licensed under CC BY 4.0. © 2017 Y. Sohtome et al.
Assembling the components of new drugs could be a lot easier thanks to RIKEN researchers’ work mapping the distribution of electrons in a catalyst.
Nickel-based catalysts are powerful agents for assembling complex organic molecules such as drugs, but they tend to be developed by trial and error. A cross-disciplinary team of RIKEN researchers has studied a nickel catalyst in more detail, developing a deep understanding of its electronic structure and resulting chemical activity1. The research paves the way for wider use of this catalyst and provides a pathway by which all catalysts could be improved.
Catalysts typically act as chemical matchmakers, bringing together two molecules and facilitating the exchange of electrons that bond them together. To make molecules such as drugs, there is an added complication. In nature, most molecules are chiral: like your left and right hands, they exist in mirror-image forms known as enantiomers. Catalysts can help guide the reactants together so that only the desired enantiomer of a drug is formed. To do this, chemists usually test a range of catalyst structures and pick the one that works best.
To develop a new nickel catalyst to selectively pair two chiral molecules in a transformation known as a [3+2] cycloaddition, Mikiko Sodeoka, Yoshihiro Sohtome and their colleagues at the RIKEN Synthetic Organic Chemistry Laboratory and the RIKEN Center for Sustainable Resource Sciencetook a different approach. By teaming up with crystallographer Daisuke Hashizume at the RIKEN Center for Emergent Matter Science, they probed the catalyst with X-rays to understand its activity by first understanding its electronic structure.
“X-ray diffraction data contains information on all electron densities in the crystal,” says Hashizume. Using this data, the team mapped the electrons spread across the complex, a process they called electron density distribution (EDD) analysis (Fig. 1). The analysis revealed a partially naked, electron-deficient d electron orbital, a feature that gives the catalyst acidic properties. They also found that one of the catalyst’s ligand arms is weakly bound and so can act as a base.
“We experimentally showed that a nickel complex has two ‘hands’, one acid and one base,” says Sohtome. The two hands work cooperatively to catalyse the cycloaddition.
“Our collaboration demonstrates that EDD analysis is a useful tool to link catalyst structure and function,” Sohtome says. The insight that the catalyst acts simultaneously as acid and base should inspire numerous other catalyst reactions. “We strongly believe that the catalyst design criteria of using chiral metal catalysts that merge acid and base functionalities, holds vast potential to design asymmetric catalysis,” he concludes.
References
- 1. Sohtome, Y., Nakamura, G., Muranaka, A., Hashizume, D., Lectard, S., Tsuchimoto, T., Uchiyama, M. & Sodeoka, M. Naked d-orbital in a centrochiral Ni(II) complex as a catalyst for asymmetric [3+2] cycloaddition. Nature Communications 8, 14875 (2017). doi: 10.1038/ncomms14875
