Aug. 11, 2006 Research Highlight Biology
Cellular scaffold plays dual role in cell division
A new spatiotemporal model of microtubule organization shows the exquisite levels at which the cell division process is controlled
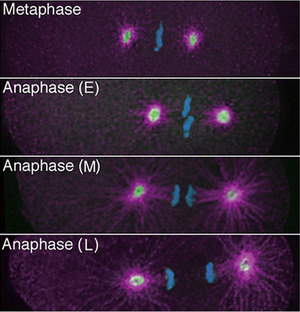 Figure 1: Fluorescently labelled cells of the worm C. elegans showing the last phases of cell division (DNA is blue; γ-tubulin is green; aurora A-kinase is pink; E is early; M is mid; L is late).
Figure 1: Fluorescently labelled cells of the worm C. elegans showing the last phases of cell division (DNA is blue; γ-tubulin is green; aurora A-kinase is pink; E is early; M is mid; L is late).
Developmental biologists at RIKEN have determined a dual role for microtubules, the basic building blocks of the internal cellular scaffold, during the final stages of cell division or cytokinesis.
During cytokinesis, cells adopt an internal organization reminiscent of the earth’s poles and equator. Spindles of microtubules connect the DNA-containing chromosomes situated along the ‘equator’ with the area around both ‘poles’, the polar cortex. As the spindles pull the chromosomes away from the equator, the cell membrane inches inwards along the equator forming a cleavage furrow. This eventually splits the cell into two daughter cells.
Working with the worm Caenorhabditis elegans, a well-known model organism for studying animal development, Asako Sugimoto and Fumio Motegi from RIKEN’s Center for Developmental Biology in Kobe, and colleagues from New York University, now propose a biphasic model for the regulation of microtubule assembly during cytokinesis. Their model, presented in Developmental Cell 1, explains cleavage furrow formation along the cell’s equator.
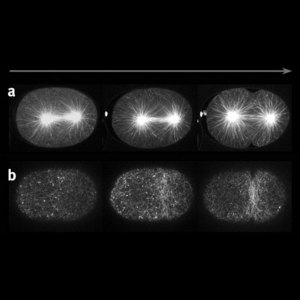 Figure 2: Cytokinesis in C. elegans. a) The mitotic spindle and b) filamentous actin of the cytoskeleton. The contractile ring forms at the spindle equator while microtubule density is high.
Figure 2: Cytokinesis in C. elegans. a) The mitotic spindle and b) filamentous actin of the cytoskeleton. The contractile ring forms at the spindle equator while microtubule density is high.
Using live imaging techniques for fluorescently labelled molecules, Sugimoto and colleagues studied the changes over time in density and location of different components of the cellular scaffold or cytoskeleton (Fig. 1). They first determined that prior to furrow formation, microtubules are enriched in the equatorial plane. All the while, filamentous actin, another component of the cytoskeleton starts forming a so-called contractile ring around the equator. As the microtubule density diminishes at the equator while increasing around the polar cortex, the contractile ring starts closing in (Fig. 2). “These observations combined with analyses using mutants and drugs revealed that the microtubules play two separate roles in the initiation of cytokinesis; one as an ‘instigator’ that localizes the contractile ring to the equator, and the other as a ‘repressor’ of the furrowing outside the equator,” explains Sugimoto.
The team then embarked on a second round of experiments to reconcile these two seemingly contradictory roles of microtubules. Applying molecular biology technologies to manipulate the activities of particular control components of microtubule assembly, they discovered that control of this process gradually ‘changes hands’ from γ-tubulin, a microtubule nucleating protein, to a second control system characterized by an increased presence of the enzyme aurora-A kinase.
“This model of dual-roles of the cytoskeleton provides an attractive alternative to existing models representing the cell cleavage process—in which single control systems fail to satisfactorily explain the different stages of cytokenesis,” notes Sugimoto.
References
- 1. Motegi, F., Velarde N.V., Piano, F., & Sugimoto, A. Two phases of astral microtubule activity during cytokinesis in C. elegans embryos. Developmental Cell 10, 509–520 (2006). doi: 10.1016/j.devcel.2006.03.001
