Feb. 23, 2007 Research Highlight Biology Chemistry
One sugar or two?
A better understanding of enzyme function using synthetic carbohydrate substrates
 Figure 1: An artistic representation of the synthethic glycan probes. © JBC/American Society for Biochemistry and Molecular Biology/ 281/ 31502 (2006)
Figure 1: An artistic representation of the synthethic glycan probes. © JBC/American Society for Biochemistry and Molecular Biology/ 281/ 31502 (2006)
Proteins are large organic molecules made up of amino acids linked together in a chain known as a polypeptide. These biological macromolecules are essential for life and are the basic components of our cellular machinery. Some of them are the catalysts that make the body’s many different biochemical reactions work and others are the scaffolds that provide structural support in the cytoskeleton.
Polypeptide chains must be folded into the correct three-dimensional structure to produce a properly functioning protein. Moreover, some misfolded proteins are known to be responsible for certain neurodegenerative diseases such as bovine spongiform encephalopathy (mad cow disease) and Alzheimer’s disease. In cells, the correct folding of polypeptides is sometimes aided by other proteins, known as chaperones.
Breaking it down
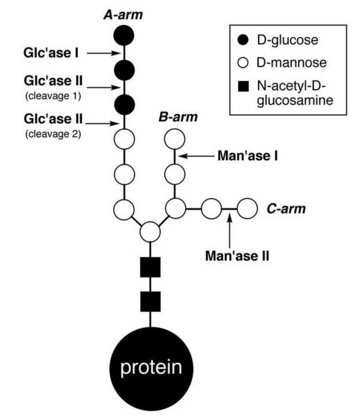 Figure 2: Glycoprotein processing in the endoplasmic reticulum. JBC/American Society for Biochemistry and Molecular Biology/ 281/ 31502–31508 (2006)
Figure 2: Glycoprotein processing in the endoplasmic reticulum. JBC/American Society for Biochemistry and Molecular Biology/ 281/ 31502–31508 (2006)
In many cases, proteins are modified by the addition of carbohydrates—sequences of sugars such as glucose and mannose—to the amino acid backbone to form glycoproteins. These ‘glycan’ chains are important in many biological processes, including protein quality control, whereby protein folding, transport and degradation (of misfolded proteins) are mediated.
Yukishige Ito from RIKEN’s Discovery Research Institute in Wako points out that, “although protein quality control is an important biological process, its molecular basis is not completely understood”. Part of the problem is that it is difficult to gain a precise understanding of the exact roles that these carbohydrate groups play because glycoproteins obtained from natural sources, such as animal cells, are often mixtures—they differ in the number and arrangement of carbohydrate groups attached to them.
In order to get a more quantitative picture of how glycan chains are involved in these cellular processes, Ito and colleagues have chemically synthesized a series of pure glycan chains and used them to study glucosidase II—an enzyme involved in glycoprotein quality control (Fig. 1). “We have established a general method to make so-called ‘high-mannose’ glycans that can be used as synthetic probes to analyze enzyme function,” comments Ito.
One step at a time
In our cells, newly made polypeptides are often modified with a glycan chain comprising 14 separate sugars, linked together in a branched structure with three arms (Fig. 2). At the end of one of these arms are three glucose sugars, and the outer two are removed sequentially by two different glycan-processing enzymes.
Glucosidase I removes the outermost glucose unit to produce a glycan known as G2M9 (named for the two glucoses and nine mannoses present in the structure), and then glucosidase II removes the next one to give a glycan containing just a single glucose unit, G1M9. This monoglucosylated structure specifically binds to calnexin and calreticulin, chaperone proteins that ensure only correctly folded proteins are transported out of the endoplasmic reticulum. The last remaining glucose residue in G1M9 can also be removed by glucosidase II, resulting in the nonglucosylated glycan M9.
In their study, published in The Journal of Biological Chemistry1, Kiichiro Totani from Ito’s laboratory and colleagues linked the synthetic glycan chains to methotrexate, a drug that is used to treat cancer by binding to an enzyme called dihydrofolate reductase (DHFR) and inhibiting its activity. This approach has many advantages, the first of which is that the methotrexate provides a spectroscopic label that can be monitored in order to follow the progress of enzymatic reactions occurring on this substrate.
Even more importantly, however, it allowed the research team to study the glycan chains in two different environments. Methotrexate is known to mimic unfolded proteins in some fashion and so the glycan-methotrexate conjugates are a good model for natural glycoproteins prior to folding. On the other hand, simply by mixing the glycan-methotrexate conjugates with DHFR (to which methotrexate strongly binds), glycan-attached proteins are formed, which serve as a model system for mature folded glycoproteins.
References
- 1. Totani, K., Ihara, Y., Matsuo, I. & Ito, Y. Substrate specificity analysis of endoplasmic reticulum glucosidase II using synthetic high mannose-type glycans. The Journal of Biological Chemistry 281, 31502–31508 (2006). doi: 10.1074/jbc.M605457200
About the Researcher
Yukishige Ito and Kiichiro Totani

Yukishige Ito was born in Kobe, Japan, in 1954. He graduated from the Faculty of Pharmaceutical Sciences, the University of Tokyo, in 1977, and obtained his PhD in 1982 from the same university. After two years postdoctoral training at the Department of Chemistry, Massachusetts Institute of Technology in Cambridge, USA, he returned to Japan as a research scientist at RIKEN, where he started his career in carbohydrate chemistry. He was promoted to senior scientist in 1996 and to chief scientist in 1998. Since then, he has been director of his own research group. His research focuses on the synthesis and functional analysis of glycoprotein-related compounds and the development of methodologies for efficient and selective synthesis of oligosaccharides.
Kiichiro Totani was born in Tokyo, Japan, in 1974, and graduated from the Department of Applied Chemistry, Keio University in 1997. After he obtained his PhD from the same university in 2002, he became a special postdoctoral researcher at the Synthetic Cellular Chemistry Laboratory at RIKEN. Since 2005, he has been working at the same RIKEN laboratory as a researcher employed by the Japan Science and Technology Agency. His research interests are synthesis and molecular recognition of sugar derivatives, including oligosaccharides and glycoproteins.
