Mar. 2, 2007 Research Highlight Biology
Two for the price of one
Two different hormones can impinge on the same signaling pathway to effect different cellular responses
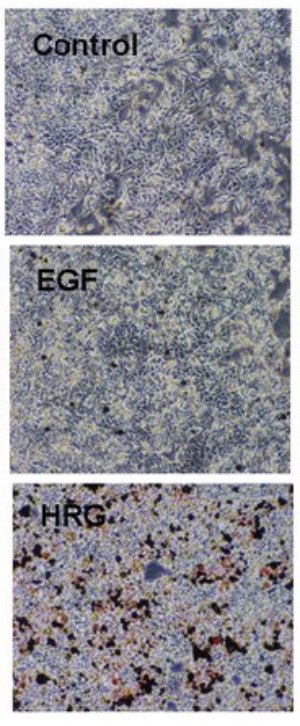 Figure 1: Treatment of breast cancer cells with heregulin (HRG) leads to cellular differentiation, as visualized by Oil Red staining of lipid droplets, whereas epidermal growth factor (ERG) does not. Copyright 2007 The American Society for Biochemistry and Molecular Biology
Figure 1: Treatment of breast cancer cells with heregulin (HRG) leads to cellular differentiation, as visualized by Oil Red staining of lipid droplets, whereas epidermal growth factor (ERG) does not. Copyright 2007 The American Society for Biochemistry and Molecular Biology
Molecular biologists at the RIKEN Genomic Sciences Center in Yokohama have taken a whole-cell systematic approach, combined with mathematical modeling, to show how different hormones can bind similar cell surface receptors and result in complementary responses by the cell1.
Cellular responses to external stimuli can be quite different. An example of this plasticity is the response of the breast cancer cell line, MCF-7, to the hormones known as epidermal growth factor (EGF) and heregulin (HRG). These hormones both play important roles in controlling cell function.
The research team, led by Mariko Hatakeyama, shows that in the MCF-7 cell line EGF evokes cell proliferation while HRG promotes differentiation (Fig. 1). This difference occurs even though both hormones bind the same type of cell-surface receptors and stimulate the same intracellular signaling pathway. However, the team also shows that EGF produces a transient activation of its receptor and its downstream signaling pathway, while that of HRG lasts much longer.
In spite of these differences in duration of activation and signaling, both hormones invoke the expression of similar sets of genes, and at similar rates, at least at early time points. However, the longer duration of signaling by HRG eventually leads to a feedback loop, such that HRG stimulates cell differentiation rather than proliferation.
The difference between EGF- and HRG-induced responses is due to a small level of change in gene activity over a large number of genes, rather than a very large change in just a few key genes. Thus, the sum of all these changes brings about a dramatic difference in later transcription and cell response. “In our view, magnitude and duration of gene expression together with signaling pathways coordinate to determine cell fate during early transcriptional relay,” says Hatakeyama. Exactly “how the difference in gene expression magnitude occurs and what may break this control” is the next research project of the team, she notes.
Hatakeyama also points out that other cell types might display this same mechanism and that it may be a general trend in biology. The analogy she likes to use is the human-chimp genome story; that is, the fact that humans and chimps are so similar in their genomes and yet so different phenotypically is probably explained by small changes over hundreds of genes—rather than a large change over a few genes. More experiments using this system-wide approach to look at other hormone responses in other cell lines should show if she is right.
References
- 1. Nagashima, T., Shimodaira, H., Ide, K., Nakakuki, T., Tani, Y., Takahashi, K., Yumoto, N. & Hatakeyama, M. Quantitative transcriptional control of ErbB receptor signaling undergoes graded to biphasic response for cell differentiation. The Journal of Biological Chemistry 282, 4045–4056 (2007). doi: 10.1074/jbc.M608653200
