Mar. 23, 2007 Research Highlight Biology
The cell link that goes with the flow
Researchers find proteins that bind cells can move
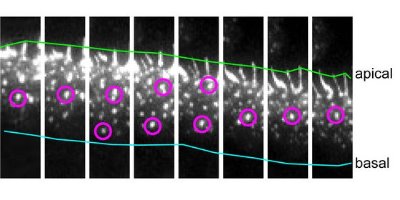 Figure 1: Time-lapse images of the movement of cadherin clusters acquired at 4-minute intervals (left to right). Two cadherin clusters are marked with magenta circles. They move up the cell junction from the basal (blue line) to the apical (green line) region. Nature Cell Biology/ Nature Publishing Group/ 9/ 93 (2006)
Figure 1: Time-lapse images of the movement of cadherin clusters acquired at 4-minute intervals (left to right). Two cadherin clusters are marked with magenta circles. They move up the cell junction from the basal (blue line) to the apical (green line) region. Nature Cell Biology/ Nature Publishing Group/ 9/ 93 (2006)
RIKEN researchers have discovered that protein molecules directly involved in binding cells into tissues can move in a coordinated, unidirectional flow. This flow is linked with the flexible actin-filament network responsible for cellular movement, and may play a significant role in cell migration and tissue remodeling.
The discovery could have clinical application as both cellular adhesion and movement where cells slide across one another without losing contact are important in physiological development, wound healing and the spread of cancer cells.
Membranes of neighboring cells in tissues are anchored to each other by means of flexible junctions involving complexes formed from three families of proteins—cadherins, catenins and actin filaments. The name cadherin is a combination of the English words ‘calcium’ and ‘adhere’ and was coined by a student of Masatoshi Takeichi, the Director of the RIKEN Center for Developmental Biology in Kobe.
The cadherin molecules extend across the membrane itself. Outside the cell, the long tails of these molecules link with cadherins of like type from an adjoining cell. Within the cell, cadherins bond with catenin molecules and through them become associated with actin filaments, though details of how this happens remain unclear. The association with actin is important, however, as its loss disrupts cell junctions.
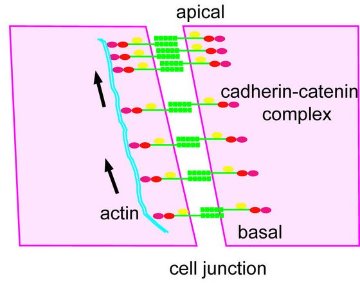 Figure 2: A schematic illustration of cadherin flow at a cell junction.
Figure 2: A schematic illustration of cadherin flow at a cell junction.
In a paper published recently in Nature Cell Biology 1, Takeichi and Yoshiko Kametani, also from the Center for Developmental Biology, describe how Kametani attached green fluorescent protein to cadherin. This allowed her to trace the movement of cadherin in epithelial cancer cells in the laboratory under the microscope.
These cells organize themselves into sheets. The zones where adjacent cells join are oblique from the lower or basal region to the upper or apical region, as if one cell were creeping under another. Kametani observed cadherin clusters forming in the basal area and moving up this incline (Fig. 1) in an organized flow along restricted routes.
When the researchers studied the molecular details of this flow, they found that the cadherin molecules in adjacent cells remained linked both to each other and to their associated catenin molecules. The whole complex moved as a unit. What’s more, by introducing mutations which disrupted the structure of the molecules in the complex, the researchers showed that the flow depended on the connection with actin (Fig. 2).
Staining studies showed that during flow the cadherin clusters maintained their association with actin filaments, which were also moving (Fig. 3). In some cases, the clusters even jumped between actin filaments. In fact, it was the actin filaments that were providing the driving force. When the researchers introduced biochemical inhibitors to stop the actin movement, the cadherin flow ceased as well.
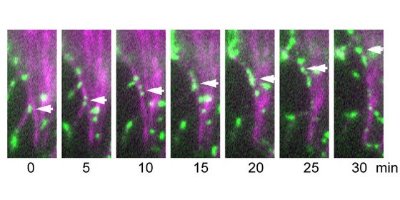 Figure 3: Time-lapse images showing the movement of cadherin clusters along actin fibers. A cell with green fluorescence protein-labeled cadherin was injected with phalloidin (tagged with a magenta fluorescence dye), which is known to bind actin. Cadherin clusters, an example of which is shown by the arrow, move along the magenta-labeled actin filament. Nature Cell Biology/ Nature Publishing Group/ 9/ 95 (2006)
Figure 3: Time-lapse images showing the movement of cadherin clusters along actin fibers. A cell with green fluorescence protein-labeled cadherin was injected with phalloidin (tagged with a magenta fluorescence dye), which is known to bind actin. Cadherin clusters, an example of which is shown by the arrow, move along the magenta-labeled actin filament. Nature Cell Biology/ Nature Publishing Group/ 9/ 95 (2006)
The fact that the cadherin complex across two cells is moving as a single unit and is motivated by actin seems to suggest that the actin filaments in the two adjacent cells are coordinated in some way. But when the researchers investigated this under the microscope, they found that actin filaments were organized and integral to the flow on one side only. The association with actin was not symmetrical.
Cadherins are present in a wide variety of cells, and therefore Kametani investigated whether cadherin flow was related to cell type. Similar directional cadherin flow was detected in some, but not all, the cell lines she observed in the laboratory. Directional, as opposed to random, flow was associated particularly with those cells that were actively moving across one another. In cells where directional flow was normally absent, it could be induced by simulating a wound in the tissue. As cell migration towards the wound began at its edges, cadherin flow commenced in the direction of the migration.
So the researchers suggest that cadherin flow may enable cells in a continuous layer to move under or over each other while retaining the ability to switch this function off once migration is no longer needed. “It seems,” Kametani says, “that cadherins may be working as a kind of clutch during cell migration that uses actin flow to provide the impetus.”
“We now want to test these ideas and uncover the biological role of this phenomenon in tissues in living organisms,” adds Takeichi, a pioneer in applying the techniques of cell biology to developmental biology. In fact, he was the first to recognize two distinct mechanisms in cell linkage, one dependent on calcium and the other not. But he is perhaps best known for his discovery and continuing work on cadherins. In addition to the research reported here, Takeichi recently turned his attention to the role of cadherins in the formation of links between neighboring nerve cells.
References
- 1. Kametani, Y. & Takeichi, M. Basal-to-apical cadherin flow at cell junctions. Nature Cell Biology 9, 92–98 (2006). 10.1038/ncb1520
About the Researcher
Masatoshi Takeichi and Yoshiko Kametani

Masatoshi Takeichi is director of the RIKEN Center for Developmental Biology in Kobe, Japan. He completed M.S. programs in biology at Nagoya University before receiving a doctorate in biophysics from Kyoto University. After attaining his PhD, he took a research fellowship at the Carnegie Institute Department of Embryology under Dr. Richard Pagano. He then returned to Kyoto University, attaining a full professorship in the Department of Biophysics, before becoming professor in the Graduate School of Biostudies at the same university. He assumed his current positions in 2000.
Yoshiko Kametani was born in 1977 in Japan, and graduated in 2001 from the Tokyo Institute of Technology, with a major in bioscience. In 2007, she received her PhD in life science from Kyoto University, under the supervision of Masatoshi Takeichi. In 2006, she joined Takeichi’s laboratory at the RIKEN Center for Developmental Biology as a research associate/scientist. In May, she moves to the Department of Biochemistry and Biophysics at the University of California, San Francisco.
