Jun. 8, 2007 Research Highlight Biology
When bad eggs are good
Eggs that fail to become fertilized can be used to obtain embryonic stem cells
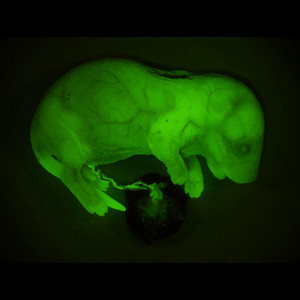 Figure 1: A mouse developed with embryonic stem cells grown from aged, fertilization-failed oocytes. Expression of the green protein GFP shows that stem cells contributed to all body tissues. © Copyright Teruhiko Wakayama 2007
Figure 1: A mouse developed with embryonic stem cells grown from aged, fertilization-failed oocytes. Expression of the green protein GFP shows that stem cells contributed to all body tissues. © Copyright Teruhiko Wakayama 2007
Embryonic stem cells can transform into many different cell types, providing a potentially limitless source of cells for medical applications. However, the process of obtaining stem cells is controversial because it involves the collection of egg cells, or oocytes, from healthy women, and the destruction of embryos that have the potential to produce offspring. Now RIKEN researchers have demonstrated an alternative, by successfully obtaining stem cells from oocytes that failed to become fertilized during IVF treatment1. Such oocytes, called ‘aged fertilization-failure’ (AFF) oocytes, would normally be discarded in clinics.
The team at the RIKEN Center for Developmental Biology in Kobe have significantly improved the process of therapeutic cloning, otherwise known as somatic cell nuclear transfer (SCNT)2. The researchers collected AFF oocytes from mice, and used SCNT to replace each oocyte nucleus with the nucleus of a non-reproductive cell taken from laboratory mice engineered to express a protein called GFP that glows green under fluorescent light. The AFF oocytes were artificially activated to develop into early-stage embryos, or blastocysts, from which stem cells were derived. In general, the success rate for developing blastocysts from AFF oocytes was lower than for fresh oocytes, but those AFF oocytes that did reach the blastocyst stage had high success in producing stem cells.
The stem cells derived from AFF oocytes had the normal arrangement of chromosomes, and when they were injected into fresh mouse embryos they contributed to the development of healthy, fertile animals. Furthermore, each entire animal appeared green under fluorescent light (Fig. 1), proving that the stem cells are capable of differentiating into all cell types in the body.
However, none of the cloned blastocysts from AFF oocytes developed to full term offspring. This suggests that AFF oocytes have deficiencies that hinder full-term development, but are sufficient for the generation of stem cells. The findings could address some ethical concerns about the method—if the blastocyst will never be able to develop, why not use it to acquire stem cells?
Project leader Teruhiko Wakayama believes that the techniques can be further improved to produce human stem cells, but some concerns will remain. “AFF oocytes are the best solution to end the ethical debate, but due to the low success rate, you must prepare a hundred AFF oocytes from hospitals for each patient,” he says. “We must improve the success rate to reduce the number of used AFF oocytes.”
References
- 1. Wakayama, S., Suetsugu, R., Thuan, N.V., Ohta, H., Kishigami, S. & Wakayama, T. Establishment of mouse embryonic stem cell lines from somatic cell nuclei by nuclear transfer into aged, fertilization-failure mouse oocytes. Current Biology 17, 120–121 (2007). doi: 10.1016/j.cub.2006.12.020
- 2. Kishigami, S., Mizutani, E., Ohta, H., Hikichi, T., Thuan, N.V., Wakayama, S., Bui, H.T. & Wakayama, T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochemical and Biophysical Research Communications 340, 183–189 (2006). 10.1016/j.bbrc.2005.11.164
