Jul. 27, 2007 Research Highlight Biology
The birth of reproduction
Researchers find key to how DNA replication begins
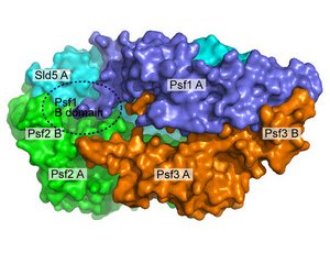 Figure 1: A model of the GINS complex showing how the subunits fit together and highlighting the critical B domain of Psf1. © Nature Structural & Molecular Biology/ Nature Publishing Group/ 14/ 399 (2007)
Figure 1: A model of the GINS complex showing how the subunits fit together and highlighting the critical B domain of Psf1. © Nature Structural & Molecular Biology/ Nature Publishing Group/ 14/ 399 (2007)
A team of Japanese molecular biologists has determined the structure of a protein complex that plays a key role in the initiation of one of the most fundamental of biological processes: the replication of DNA. The structure suggests how the complex functions, they say.
During replication, the double-stranded, helical DNA molecule is unraveled into two single strands each of which serves as a template for synthesizing a new and complementary strand of genetic material. The complete process generates two identical double-strands of DNA.
Previous studies have provided evidence that the so-called GINS complex, a macromolecular protein complex, is an important component of the molecular machinery that splits the two strands of helical DNA apart, creating what is known as a replication fork.
In a recent paper in Nature Structural & Molecular Biology1, researchers from RIKEN’s Discovery Research Institute in Wako and from Osaka University describe how they determined the crystal structure of the four subunits comprising the GINS complex and demonstrated how they fit together. Subsequent biochemical analyses in which crucial parts of some subunits were removed or altered systematically allowed the team to infer the roles of the subunits.
The four protein subunits—Sld5, Psf1, Psf2 and Psf3—are of similar size, each about 200 amino acids long. The team’s crystallographic work showed that the subunits pack together into a two-layered trapezoid structure, with Sld5 and Psf1 on one level above Psf2 and Psf3, respectively, on the other (Fig. 1). Each subunit includes two domains, A and B, through which they interact and link to each other.
The team’s biochemical analyses demonstrated that Psf1 is the most critical to the operation of the GINS complex. Its B domain must remain intact and in position for the complex to retain its full activity. While altering the B domain of Psf1’s horizontal partner Sld5 loosens the tightness with which the complex is bound and can reduce activity; loss of the B domain of Psf1 has no effect on stability of the complex but inhibits its activity completely.
The research team suggests that while the other three subunits provide a tightly bound platform to support Psf1, it is the B domain of Psf1 that interacts with and organizes other proteins to initiate the DNA replication process. In future, the group hopes to determine which protein actually binds to GINS, with the aim of eventually initiating DNA replication in vitro.
References
- 1. Kamada, K., Kubota, Y., Arata, T., Shindo, Y. & Hanaoka, F. Structure of the human GINS complex and its assembly and functional interface in replication initiation. Nature Structural and Molecular Biology 14, 388–396 (2007). doi: 10.1038/nsmb1231
