Jun. 13, 2008 Research Highlight Biology
A ‘turn-off’ that promotes reproduction
New research has revealed a tiny protein with huge responsibilities—regulating the formation of reproductive cells in the developing fly embryo
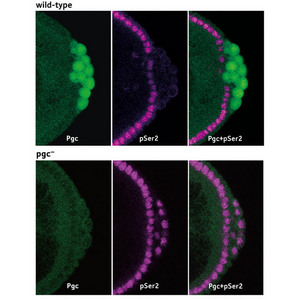 Figure 1: Drosophila embryos which have been fluorescently labeled to reveal expression of Pgc (Pgc, green) and CTD phosphorylation (pSer2, purple). In wild-type embryos (upper panels), CTD phosphorylation is eliminated in the Pgc-expressing germ cell progenitors. When pgc expression is eliminated (lower panels), CTD phosphorylation is detected throughout the embryo. Reproduced from Ref. 1 © 2008 Nature Publishing Group
Figure 1: Drosophila embryos which have been fluorescently labeled to reveal expression of Pgc (Pgc, green) and CTD phosphorylation (pSer2, purple). In wild-type embryos (upper panels), CTD phosphorylation is eliminated in the Pgc-expressing germ cell progenitors. When pgc expression is eliminated (lower panels), CTD phosphorylation is detected throughout the embryo. Reproduced from Ref. 1 © 2008 Nature Publishing Group
During embryonic development, somatic cells form the vast majority of the body’s tissues and organs, while germ cells are the foundational cells for sexual reproduction. Germ cell formation requires the global blocking of transcriptional activity, to prevent the expression of the genes responsible for inducing somatic cell differentiation.
The enzyme RNA polymerase II is the primary engine of gene transcription, but proper operation of the enzyme requires phosphorylation—the addition of a phosphate group—to a region called the carboxy-terminal domain (CTD). In some organisms, the formation of germ cells from precursors is promoted in part by the blocking of CTD phosphorylation.
Over a decade ago, Akira Nakamura—now at the RIKEN Center for Developmental Biology in Kobe—identified the RNA of the pgc gene as an essential factor for germ cell formation in the fruit fly, Drosophila melanogaster1. Based on existing evidence, this RNA was thought to act as a noncoding RNA rather than being translated into protein, and this model has persisted since then.
However, more recent work from Nakamura and colleagues has now overturned this hypothesis2. Their careful analysis of the pgc RNA sequence revealed the potential to encode a relatively tiny protein, and they were subsequently able to detect this protein within Drosophila embryo germ cell progenitors.
When expression of pgc was eliminated, CTD phosphorylation was detectable in these progenitors (Fig. 1), preventing proper germ cell formation and resulting in the development of sterile adult flies.
Nakamura and colleagues found that the Pgc protein not only interacts with P-TEFb, the protein complex responsible for CTD phosphorylation, but also appears to actively sequester it. The result is that P-TEFb can no longer associate with normally active promoters, and transcription is thereby inhibited.
“Our study is the first to establish that inhibition of P-TEFb activity is essential for germ cell specification in an intact organism,” says Nakamura. The significance of this finding is further reinforced by mounting evidence that the recruitment of P-TEFb to promoters may be one of the most important checkpoints for transcriptional regulation.
As such, the team is now actively engaged in better understanding how Pgc interacts with and sequesters P-TEFb—information that could also have valuable clinical consequences. “P-TEFb has been implicated in several serious diseases, including cardiac hypertrophy and cancer, and is therefore a potential target of therapeutic interventions,” says Nakamura, “and Pgc may be an ideal archetype for developing potent and specific P-TEFb inhibitors.”
References
- 1. Nakamura, A., Amikura, R., Mukai, M., Kobayashi, S. & Lasko, P.F. Requirement of a noncoding RNA in Drosophila polar granules for germ cell establishnment. Science 274, 2075–2079 (1996). doi: 10.1126/science.274.5295.2075
- 2. Hanyu-Nakamura, K., Sonobe-Nojima, H., Tanigawa, A., Lasko, P. & Nakamura, A. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature 451, 730–733 (2008). doi: 10.1038/nature06498
