Aug. 8, 2008 Research Highlight Biology
Choosing the right path
A new study of blood cell development could help put an end to a decade-old controversy about immune cell differentiation
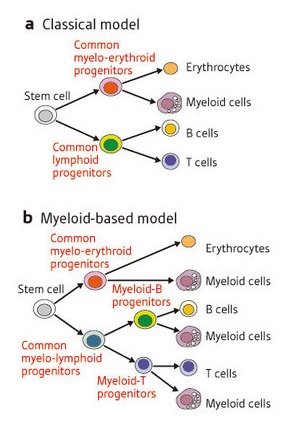 Figure 1: Two models of blood cell differentiation. (a) In the long-standing ‘classical model’, stem cells can differentiate into myelo-erythroid progenitors, which can form myeloid cells and erythrocytes (red blood cells), or CLPs, which can form B and T cells but not myeloid cells. (b) Kawamoto and colleagues’ findings support a ‘myeloid-based model’, in which CLPs are not a requisite intermediate stage. Instead, stem cells develop into common myelo-lymphoid progenitors and then into myeloid-B or myeloid-T progenitors, all of which retain the ability to form myeloid as well as lymphoid cells.
Figure 1: Two models of blood cell differentiation. (a) In the long-standing ‘classical model’, stem cells can differentiate into myelo-erythroid progenitors, which can form myeloid cells and erythrocytes (red blood cells), or CLPs, which can form B and T cells but not myeloid cells. (b) Kawamoto and colleagues’ findings support a ‘myeloid-based model’, in which CLPs are not a requisite intermediate stage. Instead, stem cells develop into common myelo-lymphoid progenitors and then into myeloid-B or myeloid-T progenitors, all of which retain the ability to form myeloid as well as lymphoid cells.
Blood contains many different cell types, all of which develop from a common stem cell precursor via a multi-stage differentiation process. T cells and B cells, immune cells that respond to foreign particles in the body, belong to a class known as lymphocytes, and have long been assumed to follow a similar developmental course. This ‘classical model’ of differentiation predicts that stem cells will differentiate into common lymphoid progenitors (CLPs), which become T and B cells, or myelo-erythroid progenitors, which form myeloid cells—such as macrophages—and red blood cells (Fig. 1a).
It was therefore quite surprising when immunologists Hiroshi Kawamoto and Yoshimoto Katsura were unable to detect CLPs from blood cell progenitors in the fetal liver1. Their 1997 study suggested a ‘myeloid-based model’ of differentiation (Fig. 1b), in which the ability to form myeloid cells is retained by all blood cell progenitors. However, another group’s subsequent discovery of putative CLPs in adult bone marrow2 left immunologists with a controversy on their hands: do fetal and adult blood cells develop along different pathways?
New work from Kawamoto’s group at the RIKEN Research Center for Allergy and Immunology, Yokohama, represents important progress towards resolving this mystery3. Kawamoto and colleagues developed a new culture system that enabled them to gauge the ability of progenitor cells from different developmental stages to form T and myeloid cells. The researchers found that these progenitors could form macrophages even at stages of differentiation where B cell formation was no longer a possibility, regardless of whether the cells were adult or fetal in origin. Similar results were obtained in live mice when these later-stage progenitor cells were grafted into the thymus.
Kawamoto believes that these findings support a myeloid-based model of development for both fetal and adult immune cells, and argues against the need for CLPs. “Many researchers have taken it [for] granted that T cells and B cells are very closely related, and thus both lineage cells [are] generated through a common pathway,” he says. “Our findings—that T cells and macrophages are generated from progenitors that have lost B cell potential—refute this dogma.”
These findings should shed new light on T cell development. “Now that we have certified that the myeloid-T bipotential stage exists in hematopoiesis, we are in the best place to start to clarify the molecular mechanisms of myeloid-T lineage commitment,” he says. “These studies will give us answers on what environmental and intrinsic factors determine the identity of T cell lineage.”
References
- 1. Kawamoto, H., Ohmura, K. & Katsura, Y. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. International Immunology 9, 1011–1019 (1997). doi: 10.1093/intimm/9.7.1011
- 2. Kondo, M., Weissman, I.L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997). doi: 10.1016/S0092-8674(00)80453-5
- 3. Wada, H., Masuda, K., Satoh, R., Kakugawa, K., Ikawa, T., Katsura, Y. & Kawamoto, H. Adult T-cell progenitors retain myeloid potential. Nature 452, 768–772 (2008). doi: 10.1038/nature06839
