Nov. 7, 2008 Research Highlight Biology
Keeping development organized
Researchers have identified a novel factor—and an unexpected mechanism—for the regulation of epithelial development
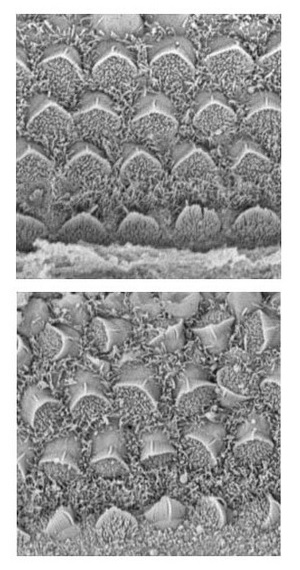 Figure 1: Electron microscope images of sensory hair cells from the inner ear. The upper panel depicts normal hair cells from a mouse with reduced expression of Cthrc1 and normal levels of Vangl2. In the lower panel, the absence of Cthrc1 expression along with reduced levels of Vangl2 leads to pronounced defects in hair cell orientation and alignment.
Figure 1: Electron microscope images of sensory hair cells from the inner ear. The upper panel depicts normal hair cells from a mouse with reduced expression of Cthrc1 and normal levels of Vangl2. In the lower panel, the absence of Cthrc1 expression along with reduced levels of Vangl2 leads to pronounced defects in hair cell orientation and alignment.
Signaling factors known as Wnts play an integral role in processes relating to body pattern formation during embryonic development. The Wnts can trigger a broad range of different signaling pathways; the specification of which particular pathway gets activated is managed by additional interactions between Wnt receptors and specific cofactors.
One process controlled by the Wnts is the planar cell polarity (PCP) pathway, which is essential to proper epithelial formation, the cells that line the surface of the skin and body cavities. “Each cell in epithelium has a polarity in the plane of the epithelial sheet—this can be easily seen in the fact that hairs are aligned in one direction,” explains Hiroshi Sasaki of the RIKEN Center for Developmental Biology in Kobe. “The PCP pathway regulates such polarity of cells.”
Sasaki’s group has been searching for novel genes involved in body pattern formation, and recently identified Cthrc1, a gene that exhibits spatial and temporal expression patterns that mirror those of known components of the PCP pathway. This led them to hypothesize that Cthrc1 may also be acting within this signaling cascade.
In order to test this model, they generated strains of mice in which the expression of Cthrc1 had been eliminated1. Disruption of this gene had no apparent effect on its own, but when Sasaki’s team further modified the mouse strain to reduce expression of the PCP signaling gene Vangl2, they observed marked abnormalities in orientation and alignment of the sensory hair cells of the inner ear (Fig. 1). Reduction of Vangl2 alone was not sufficient to cause these defects, further supporting a role for Cthrc1 in PCP.
Subsequent analysis showed that the Cthrc1 protein directly interacts with and stabilizes the complex formed by Wnts with their receptors and PCP-related co-receptors, and thereby specifically enhances PCP pathway activation while suppressing other Wnt-mediated signaling cascades. Importantly, this interaction occurs outside the cell. “Our paper reports—for the first time—an extracellular molecule involved in pathway selection by Wnt signaling molecules,” says Sasaki, “and therefore reveals a novel mechanism for pathway selection.”
Beyond these insights, however, this work also yields a new mystery—if eliminating Cthrc1 expression causes no ill effects, which protein is taking its place? “Because there are no Cthrc1-related genes in the mouse genome, structurally unrelated molecules must play similar roles in Wnt signaling,” says Sasaki. “We think that it is necessary to identify such molecules to reveal the importance of this mechanism in Wnt signaling.”
References
- 1. Yamamoto, S., Nishimura, O., Misaki, K., Nishita, M., Minami, Y., Yonemura, S., Tarui, H. & Sasaki, H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Developmental Cell 15, 23–26 (2008). doi: 10.1016/j.devcel.2008.05.007
