Dec. 26, 2008 Research Highlight Biology
Looping-the-loop
A unique model may describe the genetic switch that controls whether a T cell becomes a helper or a killer
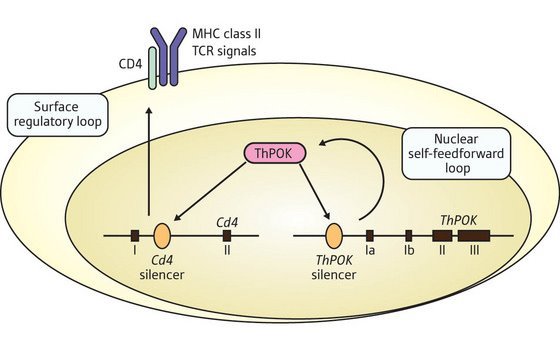 Figure 1: Regulation of ThPOK expression in helper T cells. ThPOK (magenta) reverses the silencing of the Cd4 gene as well as its own gene in a positive auto-feedforward mechanism. (TCR, T Cell Receptor; roman numerals denote segments of the Cd4 and ThPOK genes).
Figure 1: Regulation of ThPOK expression in helper T cells. ThPOK (magenta) reverses the silencing of the Cd4 gene as well as its own gene in a positive auto-feedforward mechanism. (TCR, T Cell Receptor; roman numerals denote segments of the Cd4 and ThPOK genes).
Immunologists have come a step closer to understanding the genetic controls that direct the fate of the lynchpins of the immune system—T lymphocytes.
As T lymphocytes mature, they develop into different types, or lineages, which are characterized by different cell surface markers and have different roles in the immune response.
Helper T cells, which coordinate the immune response and promote antibody production, carry the CD4 surface marker protein, and require interaction with immune proteins called MHC class II molecules during development and differentiation. Killer T cells, which are cytotoxic and capable of directly killing infected or cancerous cells, carry the CD8 surface marker protein and must interact with MHC class I molecules during development and differentiation.
But the molecular mechanisms governing lineage decision of an immature T cell, or thymocyte, expressing both the CD4 and the CD8 markers to differentiate into CD4-positive, CD8-negative T helper or CD4-negative, CD8-positive T cytotoxic lineages are largely unknown.
Now, a team led by Ichiro Taniuchi at the RIKEN Center for Allergy and Immunology in Yokohama has started to unravel some of the genetic controls directing the expression of lineage-specific genes.
A transcription factor called ThPOK has been identified in previous studies as being essential for the differentiation of MHC class II-selected thymocytes into helper T cells.
Taniuchi and colleagues used a fluorescent marker protein to visualize the expression of the ThPOK gene during the process of commitment to a specific T cell lineage1. The gene has several regulatory elements, known as enhancers and silencers, which were modified in turn to assess the impact on lineage determination.
From these results, the researchers have developed a model that suggests stimulation of the T cell receptor during the initial stage of thymocyte differentiation induces ThPOK expression by reversing the activity of the ThPOK silencer. ThPOK then antagonizes the CD4 silencer element, allowing expression of CD4, which in turn allows continued MHC class II-stimulation of the cell.
This has the effect of increasing ThPOK expression, generating a second auto-regulatory loop through the action of ThPOK to antagonize the silencer element within its own gene. The positive auto-feedforward mechanism amplifies and then stabilizes ThPOK expression in fully committed helper T cells (Fig. 1).
“Our model is unique and could be the first one that describes an auto-regulatory loop by antagonizing a transcriptional silencer within its own locus,” says Taniuchi. “I do expect that a similar mechanism may function in other lineage decision processes.”
References
- 1. Muroi, S., Naoe, Y., Miyamoto, C., Akiyama, K., Ikawa, T., Masuda, K., Kawamoto, H. & Taniuchi, I. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nature Immunology 9, 1113–1121 (2008). doi: 10.1038/ni.1650
