Mar. 13, 2009 Research Highlight Biology
Finding the sweet spot
A previously enigmatic protein has been found to play a direct role in monitoring glucose levels in the body
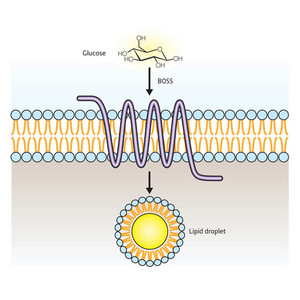 Figure 1: BOSS appears to regulate the storage of lipids within cells in response to levels of circulating glucose.
Figure 1: BOSS appears to regulate the storage of lipids within cells in response to levels of circulating glucose.
There is considerable evidence that the simple sugar glucose is more than just a source of sweetness and energy. “Scientists believe that glucose is not only a nutrient molecule, but also a signaling molecule that stimulates cells and tissues through receptor molecules,” explains Yoshio Hirabayashi of the RIKEN Brain Science Institute in Wako.
However, although researchers have identified components from a variety of glucose-responsive pathways, it has proven difficult to identify the receptors that directly bind glucose molecules and initiate these signaling circuits.
Hirabayashi’s team was investigating an unrelated signaling pathway in the fruit fly Drosophila when they recently came across an intriguing receptor molecule known as BOSS. BOSS has been previously associated with pathways related to eye development, but very little is known about its function overall, and—tantalizingly—it contains small fragments also found in the taste receptor and a sugar transport protein.
The group decided to pursue this promising lead, and their recently published findings provide strong evidence that BOSS is a direct mediator of metabolic regulation in response to glucose levels1.
BOSS is expressed primarily in the fat body, a large deposit of adipose tissue that plays an important role in sensing nutrient levels and regulating fly metabolism—in many ways, an analog of the vertebrate liver. Hirabayashi’s team also found that BOSS signaling activity was stimulated by the presence of glucose in a dose-dependent manner in cultured cells, and observed broad evidence of metabolic dysregulation in fly larvae lacking a functional boss gene. These larvae were smaller overall, and exhibited elevated levels of circulating glucose and increased lipid consumption—much like larvae with defects in insulin signaling pathways.
Together, these data suggest a direct role for BOSS in sensing and responding to glucose levels (Fig. 1). “This is the first report of a glucose-responsive receptor in multicellular organisms,” says Hirabayashi. “I believe that BOSS is one of the important factors controlling homeostasis of glucose and lipid (energy) metabolism.”
Flies offer a simple model for studying glucose metabolism, but these processes appear to also be closely conserved in higher organisms, and Hirabayashi sees these findings as a jumping point for parallel research in mammals. “In fact, mammals—including humans—have receptors homologous to BOSS,” he says. “We have knocked this gene out in mice, and I hope that these mice will tell us why the BOSS gene is so strongly conserved.”
References
- 1. Kohyama-Koganeya, A., Kim, Y.-J., Miura, M. & Hirabayashi, Y. A Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: Loss of boss causes abnormal energy metabolism. Proceedings of the National Academy of the Sciences USA 105, 15328–15333 (2008). doi: 10.1073/pnas.0807833105
