Feb. 26, 2009 Research Highlight Biology
Island hopping
Cells control interactions between two proteins with an important role in Alzheimer’s disease by stranding them on discrete membrane ‘islands’
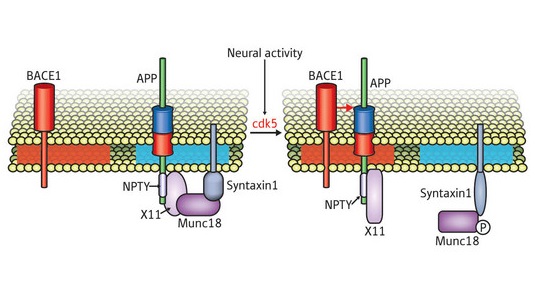 Figure 1: Cleavage dependent on neuronal activity. BACE1 and APP are normally sequestered in different types of microdomains (pink and blue boxes). This is mediated by a complex of proteins (syntaxin, munc18 and x11) that anchor APP. However, increased neuronal activity stimulates cdk5, which introduces modifications that disrupt this complex and allow APP to associate with BACE1-containing microdomains, resulting in elevated Aβ production.
Figure 1: Cleavage dependent on neuronal activity. BACE1 and APP are normally sequestered in different types of microdomains (pink and blue boxes). This is mediated by a complex of proteins (syntaxin, munc18 and x11) that anchor APP. However, increased neuronal activity stimulates cdk5, which introduces modifications that disrupt this complex and allow APP to associate with BACE1-containing microdomains, resulting in elevated Aβ production.
The earliest known stage in the pathology of the neurodegenerative disorder Alzheimer’s disease involves the accumulation of deposits of amyloid-β peptide (Aβ) in the brain. Aβ is a byproduct of cleavage of amyloid precursor protein (APP), a membrane protein that is particularly abundant at neuronal synapses.
Both APP and BACE1—the enzyme that cleaves it—are thought to associate with microdomains, patches containing specific lipid subtypes and membrane proteins that form discrete islands adrift in the plasma membrane. What remains unclear, however, is the role of these microdomains in the regulation of APP cleavage and Aβ production.
In order to better understand this process, Nobuyuki Nukina and his colleagues at the RIKEN Brain Science Institute in Wako set about isolating and characterizing microdomains containing APP1. They have found that APP normally forms part of a multi-protein complex that appears to stabilize its inclusion in certain types of microdomains, but BACE1 appears to be segregated to a completely distinct subset of microdomains, suggesting the need for an active process that reunites these two proteins in order to produce Aβ. “It is necessary for the substrate, APP, and enzyme, BACE1, to be in the same microdomain,” explains Nukina.
Previous studies have indicated that increased neuronal activity is directly correlated with increased production of Aβ, and subsequent experiments by Nukina’s group helped to reveal the chain of events underlying one mechanism by which this may occur. They found that prolonged synaptic activity stimulates a protein called cdk5, which introduces a chemical modification to the APP-containing protein complex; this modification leads to disruption of the multi-protein complex that helps anchor APP to its microdomain, releasing it and enabling it to associate instead with BACE1-containing microdomains—and thus undergo cleavage (Fig. 1).
In addition to providing new insights into the mechanisms involved in the initial stages of Alzheimer’s pathology, these findings may further illuminate the functional importance of the various microdomain types. “It has been reported that different microdomains in the membrane contain particular protein complexes, but the significance of this has not been known,” says Nukina. “Our report suggests that microdomain switching of APP to BACE1-containing microdomains regulates APP cleavage, [and] similar mechanisms may exist in the processing of other proteins.”
References
- 1. Sakurai, T., Kaneko, K., Okuno, M., Wada, K., Kashiyama, T., Shimizu, H., Akagi, T., Hashikawa, T. & Nukina, N. Membrane microdomain switching: a regulatory mechanism of amyloid precursor protein processing. The Journal of Cell Biology 183, 339–352 (2008). doi: 10.1083/jcb.200804075
