Jan. 8, 2010 Research Highlight Biology
Sights set on immunization target
Revelation of key elements of how the digestive system defends the body broadens the scope for oral vaccines
A RIKEN-led research team has unraveled the molecular details of a key mechanism of the immune system in the gut. The work opens the way to new possibilities for developing versatile, inexpensive vaccines that are swallowed, rather than injected.
“The description of this molecular pathway fills a gap in our understanding of the immune response of the digestive system,” says team leader Hiroshi Ohno. “And it provides molecular targets for compounds taken orally, and therefore offers the hope of an easy-to-administer, cost-effective weapon against infectious diseases and allergies.”
Intestinal armory
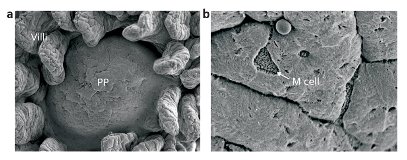 Figure 1: Two scanning electron micrographs of a murine Peyer’s patch (PP). In the image of higher magnification (right), an M cell is visible. Left: Reproduced, with permission, from Experimental Medicine 24, 3112–3121 (2006) © (2009) Yodosha Right: Reproduced, with permission, from Japanese Journal of Clinical Immunology 29, 16–26 (2006) © (2009) The Japan Society for Clinical Immunology
Figure 1: Two scanning electron micrographs of a murine Peyer’s patch (PP). In the image of higher magnification (right), an M cell is visible. Left: Reproduced, with permission, from Experimental Medicine 24, 3112–3121 (2006) © (2009) Yodosha Right: Reproduced, with permission, from Japanese Journal of Clinical Immunology 29, 16–26 (2006) © (2009) The Japan Society for Clinical Immunology
The mouth is the most significant entry point into the body for pathogens, allergens and poisons. Most of what comes into the body through the mouth moves through the digestive system. As a result, the lining of the gut houses the largest part of the entire immune system. It protects the body from disease organisms and foreign particles by secreting vast amounts of antibodies in the form of Immunoglobulin A.
Antibodies combine with, neutralize and mark out their targets, known as antigens, for future attack by other parts of the immune system. In order to form antibodies targeting specific foreign particles or organisms in the digestive system, the antigens are presented to immature dendritic cells in Peyer’s patches—organized bodies of immune system tissue found beneath the epithelial cells that line the gut. It has long been suspected that specialized microfold or M cells (Fig. 1) in the epithelium are involved with the process of moving such antigens from the gut cavity to the immune system cells underneath. Until now, however, the molecular details of the process have remained a mystery.
Investigative artillery
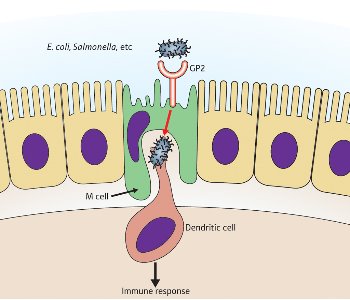 Figure 2: A schematic diagram depicting the role of GP2 in the immune response of the gut. © (2009) RIKEN
Figure 2: A schematic diagram depicting the role of GP2 in the immune response of the gut. © (2009) RIKEN
Using the latest techniques of micro-dissection, microarray analysis, staining, microscopy and molecular genetics to investigate, Ohno and colleagues from the RIKEN Research Center for Allergy and Immunology, Yokohama, collaborated with biologists from Yokohama City University, several other Japanese universities and Stanford University in the US.
They uncovered a particular receptor molecule on M cells that stimulates the immune response by binding to a protein on the hair-like projections or pili of bacteria such as Escherichia coli and Salmonella 1. The epithelial M cells engulf foreign bodies in the gut cavity, surrounding them with the cell membrane. These membrane-sealed packages or vesicles are then passed through the body of the M cell to waiting immune system cells in a pocket on the underside (Fig. 2).
The researchers speculated that the M cells may have specific molecular receptors to bind to antigen targets. So, working in mice, Ohno and colleagues used the analytical technique known as microarray technology to scan the genome for receptor molecules specific to M cells. Glycoprotein 2 (GP2) was one such molecule and the microarray analysis showed it was highly associated with epithelium close to Peyer’s patches. When they stained both GP2 and M cells, they discovered that not only was GP2 restricted to M cells on Peyer’s patches in the gut, but that it was also found on M cells associated with immune system tissues in other parts of the body in humans as well as mice. In fact, GP2 is a universal marker for M cells.
Moving in closer, the researchers employed electron microscopy to determine where GP2 was distributed on the M cells. They found it was localized on the membrane facing the gut cavity. By using an antibody that binds only to GP2, they discovered that some was incorporated into vesicular structures in the body of the M cells. This provided further evidence that the receptors were involved in the mechanism for transporting antigens to the immune system cells beneath.
Profiling glycoprotein 2
The next step was to determine what compound or compounds linked to the GP2 receptor. By assessing the structure of GP2, the researchers discovered that it resembles a protein in the kidney that binds to pathogenic E. coli bacteria. Thus, they reasoned that GP2 might also be associated with bacteria in the gut.
Ohno and colleagues then experimented by mixing GP2 with E. coli, and found that it bound to the protein FimH on the bacterial pili. In fact, GP2 binds only to bacteria that carry FimH, and not to bacteria that lack this protein, such as Pseudomonas and Listeria.
Using an intact mouse intestine, the researchers tracked E. coli expressing green fluorescent protein. GP2 accumulated around the bacteria, which then could be followed moving inside the M cells. More than 90% of the bacteria transported through the M cells are captured by dendritic cells. They also showed that this process was severely hampered in E. coli lacking FimH or mice lacking GP2.
Fortified by FimH
Finally, the research team tested the ability of bacteria with and without FimH to induce the immune response in mice. They used a particular type of Salmonella modified to carry a fragment of inactivated toxin used as an antigen in tetanus vaccines. Those bacteria carrying FimH stimulated a much stronger immune response than those without, which the team showed was not the result of any inherent deficiency in the mouse immune system.
“It is reasonable to assume there are other molecules on the M-cell surface responsible for binding and uptake of bacteria lacking FimH,” Ohno says. “This is one of the projects we are now working on. Another is to screen for small compounds that bind tightly to GP2. We can then use these molecules to target to GP2 on M cells in an effort to develop efficient oral vaccines.”
References
- 1. Hase, K., Kawano, K., Nochi, T., Pontes, G.S., Fukuda, S., Ebisawa, M., Kadokura, K., Tobe, T., Fujimura, Y., Kawano, S., et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 462, 226–232 (2009). doi: 10.1038/nature08529
About the Researcher
Hiroshi Ohno
Hiroshi Ohno was born in Tokyo, Japan, in 1958. He graduated from the School of Medicine, Chiba University, in 1983, and obtained his PhD in 1991 from the same university. He then became an assistant professor of the School of Medicine, and was promoted to associate professor in 1997. He spent three years from 1994 to 1997 as a visiting scientist at the National Institute of Child Health and Human Development, National Institutes of Health in the USA. In 1999, he became a full professor at the Cancer Research Institute of Kanazawa University. He joined the RIKEN Research Center for Allergy and Immunology as team leader in 2002, where his research focuses on mucosal immunology, and in particular the role of epithelial cells in the development of the mucosal immune system, mucosal antigen uptake and initiation of mucosal immune responses.
