Apr. 9, 2010 Research Highlight Biology
Insights to air supply
The structure of a key membrane protein expressed in red blood cells could reveal how oxygen supply to tissues is regulated
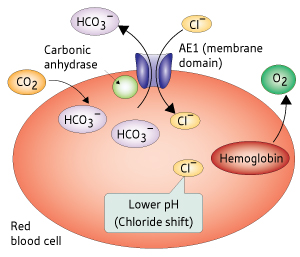 Figure 1: Schematic showing the exchange of bicarbonate (HCO3¯) and chloride (Cl¯) ions via AE1, which promotes the release of oxygen from hemoglobin by lowering the pH within the red blood cell. © 2010 Teruhisa Hirai
Figure 1: Schematic showing the exchange of bicarbonate (HCO3¯) and chloride (Cl¯) ions via AE1, which promotes the release of oxygen from hemoglobin by lowering the pH within the red blood cell. © 2010 Teruhisa Hirai
Teruhisa Hirai of the RIKEN SPring-8 Center, Harima, and colleagues have obtained structural information about the physiologically important protein called erythrocyte anion exchanger 1 (AE1)1.
As well as being expressed in the kidney, AE1 is the most abundant protein in the membrane of red blood cells, or erythrocytes, and helps prevent tissue damage by regulating oxygen supply.
Carbon dioxide, produced when food is burnt for energy, is released into the bloodstream and, on encountering erythrocytes, is converted into bicarbonate (HCO3¯) by the enzyme carbonic anhydrase bound to AE1. In a crucial step—the ‘chloride shift’—chloride is exchanged for HCO3¯ across the erythrocyte membrane via AE1. This lowers erythrocyte pH, resulting in the regulated release of oxygen from hemoglobin (Fig. 1).
Despite its importance, there is limited structural information about AE1, particularly the membrane-spanning domain. “Like many membrane proteins, AE1 is very fragile, making structural information difficult to obtain,” says Hirai.
Naotaka Hamasaki and colleagues at Nagasaki International University and Kyushu University purified AE1 stably with the membrane domain fixed in an outward-open conformation, and good two-dimensional crystals were prepared at RIKEN by Tomohiro Yamaguchi. “These were critical steps for structural analysis,” explains Hirai.
The researchers merged 31 images, each taken from a different angle, to produce a three-dimensional image. Interestingly, they found structural similarities between AE1 and a bacterial transporter protein related to a class of chloride channels called ‘ClC channels’ found in animals, including humans.
DNA sequence information is available for the anion exchanger and ClC gene families, but the researchers are the first to uncover structural similarities between the encoded proteins.
ClC channels of mammals conduct chloride ions and are involved in regulating the electrical excitation of skeletal muscles. The bacterial protein, however, functions as a transporter, exchanging chloride ions and protons across the bacterial membrane.
“AE1 has a putative chloride binding site similar to that of the bacterial ClC protein, although this is yet to be proven biochemically,” explains Hirai.
The observed resemblance between AE1 and ClC should help address the chloride transport mechanism, which is not well understood for either family.
“We need to improve the resolution of the current outward-open conformation structure of AE1 and solve the structure of the inward-open conformation to understand the conformational change during transport,” Hirai notes.
AE1 mutations are associated with the human genetic disorders Southeast Asian ovalocytosis and distal renal tubular acidosis, so structural knowledge of AE1 might eventually lead to treatments.
References
- 1. Yamaguchi, T., Ikeda, Y., Abe, Y., Kuma, H., Kang, D., Hamasaki, N. & Hirai, T. Structure of the membrane domain of human erythrocyte anion exchanger 1 revealed by electron crystallography. Journal of Molecular Biology 397, 179–189 (2010). doi: 10.1016/j.jmb.2010.01.027
