Nov. 5, 2010 Research Highlight Biology
Replacing faulty neurons
An effective method for generating cerebellar neurons could lead to new treatments for movement disorders
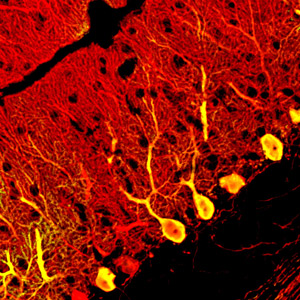 Figure 1: Purkinje neurons (yellow) generated from embryonic stem cells integrate into the cerebellum (red) when transplanted into the fetal mouse brain. Reproduced from Ref. 1 © 2010 K. Muguruma et al.
Figure 1: Purkinje neurons (yellow) generated from embryonic stem cells integrate into the cerebellum (red) when transplanted into the fetal mouse brain. Reproduced from Ref. 1 © 2010 K. Muguruma et al.
Researchers from the RIKEN Center for Developmental Biology, Kobe, have shown that neurons called Purkinje cells can not only be generated from embryonic stem (ES) cells, but can also become fully integrated into existing neuronal circuits when transplanted into the brains of mouse fetuses1.
Purkinje cells are the largest neuronal subtype in the mammalian brain, and their output in the brain region called the cerebellum controls balance, co-ordination and movement.
Yoshiki Sasai and his colleagues cultured ES cells and then treated them at different times with the hormone insulin, the naturally occurring chemical cyclopamine, and a protein called fibroblast growth factor 2, which normally induces the differentiation of Purkinje cells at a specific location in the developing hindbrain.
This treatment caused the ES cells to express genes that are specific for Purkinje cells, and then to differentiate into mature neurons with the extensive, two-dimensional dendritic tree and electrical properties that are characteristic of Purkinje cells. They found that the differentiation of the cells recapitulate the events that take place during neural development. The Purkinje cell-specific genes were expressed in the same sequence as in the embryo, and the immature cells exited the cell cycle, or stopped dividing, on a timescale comparable to that of the neurons in the developing cerebellum.
Sasai and colleagues then separated immature Purkinje cells from the ES cell cultures, and transplanted them into the brains of embryonic mice, injecting approximately 10,000 cells into each animal. They found that the transplanted cells integrated effectively into their proper location within the circuitry of the cerebellum (Fig. 1). The majority began to express Purkinje cell genes between 1 to 4 weeks after transplantation, and then differentiated into mature neurons, each with a long axon projecting down into the deep cerebellar nuclei.
The methods of Sasai and his team significantly improve on earlier methods for generating Purkinje cells from ES cell cultures. By successfully reproducing the microenvironment of the developing cerebellum, they generated up to 30-fold more Purkinje cells than previous methods.
These results therefore raise the possibility of developing cell transplantation therapies the cerebellar ataxias, a group of movement disorders characterized by severe motor in-coordination, which occur because of Purkinje cell degeneration.
“As a next step, we are attempting to generate Purkinje cells from human ES cells,” says Sasai. “This technology would be useful in establishing an in vitro disease model for spinocerebellar ataxia, to investigate its pathogenesis and to explore the possibility of gene therapy for this genetic disease.”
References
- 1. Muguruma, K., Nishiyama, A., Ono, Y., Miyawaki, H., Mizuhara, E., Hori, S., Kakizuka, A., Obata, K., Yanagawa, Y., Hirano, T. & Sasai, Y. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nature Neuroscience 13, 1171–1180 (2010) doi: 10.1038/nn.2638
