May 13, 2011 Research Highlight Biology
The beginnings of the brain
A single protein is sufficient to switch on the various genes that kick off the development of the embryonic nervous system
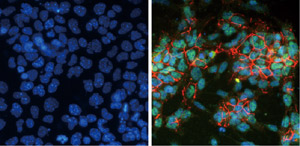 Figure 1: mES cells cultured in serum (left) are exposed to diverse factors that generally inhibit neural development. However, forced overexpression of Zfp521 in serum-exposed mES cells strongly induces production of Sox1 (green) and N-cadherin (red), two proteins closely associated with neural differentiation (right). Blue stain indicates cell nuclei. Reproduced from Ref. 1 © 2011 Daisuke Kamiya et al.
Figure 1: mES cells cultured in serum (left) are exposed to diverse factors that generally inhibit neural development. However, forced overexpression of Zfp521 in serum-exposed mES cells strongly induces production of Sox1 (green) and N-cadherin (red), two proteins closely associated with neural differentiation (right). Blue stain indicates cell nuclei. Reproduced from Ref. 1 © 2011 Daisuke Kamiya et al.
All of the tissues and organs of the body arise from one of three embryonic precursors: the ectoderm, mesoderm and endoderm. The ectoderm contributes to several tissues, including the nervous system and the skin, but some studies have suggested that development into neurons requires nothing more than the absence of specific inhibitory signals.
This phenomenon has led biologists to formulate what is called the ‘neural default model’. “The simplest interpretation of the neural default model is that the neural fate is a ‘left-over’ choice, passively determined by the elimination of other pathways of differentiation,” explains Yoshiki Sasai of the RIKEN Center for Developmental Biology in Kobe. This model fails to address the identities of the factors that actively drive neuronal development, but new findings from Sasai and colleagues have spotlighted a single protein that appears to set this process into motion1.
His team had previously designed a culture system that promotes neural differentiation of mouse embryonic stem (mES) cells2, and they used this technique to identify genes that are specifically switched on in these cells. They identified one intriguing candidate, Zfp521, which activated several other genes involved in neural development, even when the mES cells were cultured in the presence of factors that would normally curb this process (Fig. 1).
When Sasai and colleagues examined expression in developing mouse embryos, they noted that the spatial and temporal distribution of Zfp521 activity closely mirrored known sites of neural differentiation. Likewise, early stage mouse embryos injected with mES cells in which Zfp521 expression was abrogated largely failed to incorporate these cells into the developing nervous system. By systematically identifying the genes whose expression is disrupted in the absence of Zfp521, the researchers were able to determine that this gene acts as a driver for the maturation of ectodermal cells into neuroectoderm, the developmental stage that immediately precedes formation of actual neural progenitors.
“The most important message of this study is that the neural fate is acquired by an active determination process,” says Sasai. Understanding how this developmental switch works could ultimately provide scientists with a powerful tool for efficiently transforming human stem cells into mature nervous tissue suitable for experimental use or even transplantation, although it remains to be determined whether human ES cells obey the exact same principles. “We have preliminary data showing a conserved essential role for Zfp521 in both species,” says Sasai, “but we need to analyze the similarities and differences in greater depth.”
References
- 1. Kamiya, D., Banno, S., Sasai, N., Ohgushi, M., Inomata, H., Watanabe, K., Kawada, M., Yakura, R., Kiyonari, H., Nakao, K. et al. Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 470, 503–509 (2011). doi: 10.1038/nature09726
- 2. Watanabe, K., Kamiya, D., Nishiyama, A., Katayama, T., Nozaki, S., Kawasaki, H., Watanabe, Y., Mizuseki, K. & Sasai, Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nature Neuroscience 8, 288–296 (2005). doi: 10.1038/nn1402
