Mar. 2, 2012 Research Highlight Biology
Great leap forward for regenerative medicine
Recent success in growing a functional pituitary gland in a laboratory culture will advance regenerative medicine and stem-cell therapies for hormonal disorders
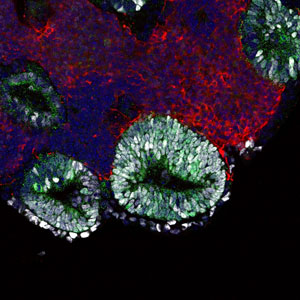 Figure 1: A micrograph of an embryonic pituitary gland that self-formed in an embryonic stem-cell aggregate on day 13 of culture. Reproduced from Ref. 1 © 2012 Yoshiki Sasai
Figure 1: A micrograph of an embryonic pituitary gland that self-formed in an embryonic stem-cell aggregate on day 13 of culture. Reproduced from Ref. 1 © 2012 Yoshiki Sasai
Embryonic stem cells grown in a laboratory culture can organize themselves into a partial pituitary gland that is fully functional when transplanted into mice, a team of researchers led by Yoshiki Sasai of the RIKEN Center for Developmental Biology reports1. These researchers developed a novel cell-culture technique for growing stem cells in three-dimensional floating clusters. They had previously shown that stem cells grown in this way can organize themselves into functional eye and brain tissue2,3.
In their study on the pituitary gland, the researchers used their technique to grow mouse stem cells, and then altered the culture conditions to recapitulate the embryonic environment that gives rise to the anterior pituitary, or adenohypophysis.
The adenohypophysis contains cells that synthesize and secrete five hormones under the control of the hypothalamus. After release into the blood stream, these hormones play multiple roles in the body, including regulation of growth, blood pressure, metabolism and sex-organ function. Development of the adenohypophysis requires interaction between two types of tissue, leading to the formation of a small pouch that pinches off from the area of the embryo and forms the mucosa in the mouth.
When the researchers stimulated stem-cell clusters with specific signaling molecules, they generated both tissue types that separated naturally into layers. Cells at the interface between the two layers then spontaneously formed oval-shaped pouch-like structures (Fig. 1), before differentiating into four types of precursors, each of which began to synthesize and secrete a different hormone.
Sasai and colleagues transplanted the clusters into the kidneys of mice whose pituitaries had been surgically removed. Normally these mice would die two months post-surgery, but the transplanted cells rescued the animals by normalizing the levels of hormones in the bloodstream.
The research could open new avenues for stem-cell therapies to treat hormonal disorders. It also represents a significant advance in using stem cells to generate complex three-dimensional structures: it represents a step towards growing fully functional organs in the laboratory.
This technique has a straightforward medical application in treating human patients with pituitary deficiencies. “Growth hormone deficiency could be also targets of the cell therapy,” Sasai notes. Eventually, it could be possible to grow functional neural tissue for transplantation into the human brain. For example, the ability to grow cerebellar tissue containing Purkinje cells could be useful for treating cerebellar atrophy.
“Regenerative medicine is surely proceeding in this direction,” says Sasai. “We are now developing computer-based models and simulations to facilitate the design of more complex organs.”
References
- 1. Suga, H., Kadoshima, T., Minaguchi, M., Ohgushi, M., Soen, M., Nakano, T., Takata, N., Wataya, T., Muguruma, K., Miyoshi, H., et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011). doi: 10.1038/nature10637
- 2. Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okura, S., Sekiguchi, K., Adachi, T. & Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011). doi: 10.1038/nature09941
- 3. Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., Kawada, M., Yonemura. S., Matsumura, M., Wataya, T., Nishiyama, A., Muguruma K. & Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). 10.1016/j.stem.2008.09.002
