Mar. 16, 2012 Research Highlight Chemistry
Double chemical action yields double success
The dynamic equilibrium between two reactive silicon compounds provides chemists with improved tools for synthesizing optically and electronically active molecules
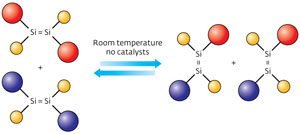 Figure 1: A cross-over reaction between dibromo-disilene compounds substituted with two types of ‘Rind’ ligands (red and blue spheres) can take place at room temperature in solution without using metal catalysts (yellow spheres, bromine). © 2012 Tsukasa Matsuo et al.
Figure 1: A cross-over reaction between dibromo-disilene compounds substituted with two types of ‘Rind’ ligands (red and blue spheres) can take place at room temperature in solution without using metal catalysts (yellow spheres, bromine). © 2012 Tsukasa Matsuo et al.
Molecules containing silicon double bonds, or disilenes, can be nearly twice as responsive to light as double-bonded hydrocarbons—a feature that makes them irresistible to researchers developing novel devices such as organic light-emitting diodes. But because disilenes are difficult to isolate and tend to polymerize, chemists struggle to control them with their usual synthetic tricks. Now, Kohei Tamao and colleagues from the RIKEN Advanced Science Institute in Wako have discovered a unique halogen-substituted disilene complex that makes assembling advanced conjugated materials easier than ever before1.
Halogen elements such as chlorine or bromine can boost the synthetic capabilities of many molecules once attached to their frameworks. Techniques known as substitution reactions can then switch the halogens for other groups, such as aromatic species. However, chemists have scarcely studied halogenated disilenes because theoretical calculations indicate that they are inherently volatile.
Recently however, Tamao and colleagues developed compounds that are extraordinarily adept at stabilizing disilenes2. Known as ‘Rind’ ligands, these molecules have a unique fused-ring structure that locks silicon double bonds into place. They also have chemically tunable side chains that optimize compatibility with a variety of substrates and solvents. Based on these capabilities, Tamao and team postulated that their technique could capture the halogenated targets.
Experiments proved that their instincts were correct: combining a Rind-protected bromine–silicon precursor with a reducing agent successfully produced the sought-after dibromo-disilene crystals. But closer examination of the new product’s reactivity revealed a surprise. Simply mixing it with an acetylene derivative caused the disilene to cleave in half and join to both sides of the carbon triple bond, producing a triangle-shaped unsaturated ring.
According to co-author Tsukasa Matsuo, this reaction provided strong evidence that the halogenated disilene could easily dissociate. To confirm this behavior, Katsunori Suzuki, another co-author, dissolved two dibromo-disilenes, each protected by a different Rind ligand, into solution. After one day at room temperature, the researchers observed an extraordinary event: the spontaneously cleaved fragments, known as bromo-silylenes, had reconnected into new disilenes containing both Rind ligands (Fig. 1). This type of ‘cross-over’ reaction, also known as olefin metathesis, is extremely useful to chemists and normally requires expensive metal catalysts to proceed.
The researchers exploited the synthetic potential of the dynamic dibromo-disilenes by capturing the reactive silylene fragment with a base, and then used this complex to construct aromatic-substituted conjugated silicon molecules inaccessible through other techniques. “These results open a new platform for development of functional disilene materials and devices,” says Matsuo.
References
- 1. Suzuki, K., Matsuo, T., Hashizume, D. & Tamao, K. Room-temperature dissociation of 1,2-dibromodisilenes to bromosilylenes. Journal of the American Chemical Society 133, 19710–19713 (2011). doi: 10.1021/ja209736d
- 2. Matsuo, T., Suzuki, K., Fukawa, T., Li, B., Ito, M., Shoji, Y., Otani, T., Li, L. Kobayashi, M., Hachiya, M. et al. Synthesis and structures of a series of bulky “Rind-Br” based on a rigid fused-ring s-hydrindacene skeleton. Bulletin of the Chemical Society of Japan 84, 1178–1191 (2011). doi: 10.1246/bcsj.20110090
