May 18, 2012 Research Highlight Biology
Development on an uneven keel
Stem cells of the fruit fly’s central nervous system shed light on the mechanism that controls asymmetrical division
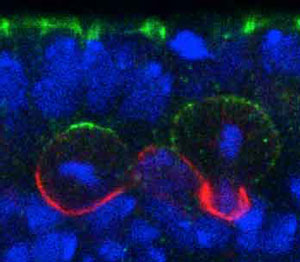 Figure 1: Neuroblasts localize polarity complexes (green) on the epithelial side (top) and divide perpendicular to the epithelium in a normal Drosophila embryo. Reproduced, with permission, from Ref. 1 © 2012 Elsevier Inc.
Figure 1: Neuroblasts localize polarity complexes (green) on the epithelial side (top) and divide perpendicular to the epithelium in a normal Drosophila embryo. Reproduced, with permission, from Ref. 1 © 2012 Elsevier Inc.
Animals consist of many distinct cell types, all of which originate during development from a single cell: the fertilized egg. To generate this vast cellular diversity, the egg and its descendants must divide unevenly to produce new cells with different fates. Nowhere is this process more important than in the central nervous system, where the asymmetric division of neural stem cells called neuroblasts contributes to the profusion of neurons and glial cells.
Several proteins assemble into so-called ‘polarity complexes’ that localize at one end of the neuroblast to help guide this unbalanced division (Fig. 1). But the mechanism that controls the orientation of these complexes has remained elusive. Now, a team of RIKEN biologists has discovered the master regulator that directs how these proteins are laid down in the neuroblasts of developing fruit fly, or Drosophila, embryos1.
Fumio Matsuzaki and his colleagues at the RIKEN Center for Developmental Biology, Kobe, screened various mutant Drosophila embryos for defects in neuroblast polarity. They uncovered an important player in this process: a gene called ‘trapped in endoderm 1’ (Tre1), which encodes a transmembrane receptor protein. In a series of experiments with fly strains in which they deleted the Tre1 gene, the researchers showed that this receptor is necessary to orient the polarity of the protein complexes in a perpendicular direction relative to the neighboring epithelial cell layer.
Further dissections of protein–protein interactions revealed that Tre1 recruits and orthogonally orients a critical polarity complex, known as Par, through a cascade of apically localized protein intermediaries. First, Tre1 activates a subunit of an important signal transducing molecule to recruit the protein Pins, which regulates spindle orientation. Another protein, called Inscuteable, then acts as a molecular link between Pins and Par to ensure that every component is in the proper location.
“The Par-complex is known to regulate the formation of cell polarity in various cell types including stem cells and neurons,” explains team member and co-author Shigeki Yoshiura. “So this process might be involved in the orientation of the polarity of various cell types during development.”
With Tre1 emerging at the top of the hierarchy controlling the orientation of polarity complexes in the neuroblast, Matsuzaki and colleagues are turning their attention to finding its regulator. “We still do not know which molecule or molecules act as the extrinsic signal from epithelial cells,” Yoshiura says. The RIKEN team is also investigating whether this mechanism is conserved through evolution and is applicable to mammalian neural stem cells.
References
- 1. Yoshiura, S., Ohta, N. & Matsuzaki, F. Tre1 GPCR signaling orients stem cell divisions in theDrosophilacentral nervous system. Developmental Cell 22, 79–91 (2012). doi: 10.1016/j.devcel.2011.10.027
