Jun. 8, 2012 Research Highlight Biology
Algal proteins light the way
Recently published insight into the structure of a light-powered protein may lead to more effective tools for probing brain function
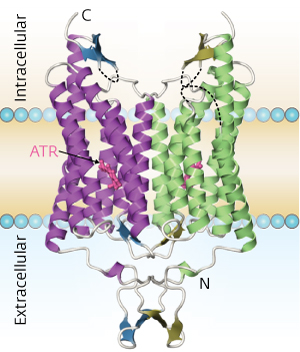 Figure 1: The structure of a chimeric channelrhodopsin protein (C1C2), a combination of elements of ChR1 and ChR2 from C. reinhardtii. The small pink structure represents all-trans retinal (ATR), a light-reactive molecule bound to C1C2 that plays an essential role in this protein’s activation. Reproduced from Ref. 1 © 2012 Hideaki Kato et al.
Figure 1: The structure of a chimeric channelrhodopsin protein (C1C2), a combination of elements of ChR1 and ChR2 from C. reinhardtii. The small pink structure represents all-trans retinal (ATR), a light-reactive molecule bound to C1C2 that plays an essential role in this protein’s activation. Reproduced from Ref. 1 © 2012 Hideaki Kato et al.
Channelrhodopsins (ChRs) are remarkable proteins that respond to specific wavelengths of light by allowing ions to cross the cell membrane, a mechanism that makes them useful for manipulating ion-driven processes in the brain. Akin to cellular-scale power switches, ChRs allow scientists to selectively switch on individual neurons or neural circuits with a flash of laser light, even in live and alert animals. These valuable tools could soon become even more useful thanks to an international collaboration at the RIKEN SPring-8 Center in Harima that has unveiled the fundamental structure of these proteins1
“Researchers have engineered ChR variants with improved properties, including ion selectivity, kinetics and absorption spectrum, but these approaches were limited by the lack of the structural information about ChR,” explains lead author Hideaki Kato, a researcher in senior author Osamu Nureki’s laboratory at the University of Tokyo. X-ray crystallography is a powerful tool for mapping the three-dimensional structure of proteins, but ChRs had proved a tricky target. Since they are difficult to produce in useful quantities and hard to crystallize, Kato and colleagues engineered a more stable hybrid chimera protein composed of parts from the closely related ChR1 and ChR2 proteins from the alga Chlamydomonas reinhardtii.
The researchers used the powerful x-ray source at the RIKEN SPring-8 Center to generate a high-quality structure of the entire light-responsive segment of ChR (Fig. 1). “The RIKEN beamline, which started operation in May 2010, is highly effective for structure determination from tiny protein crystals,” says SPring-8 scientist and co-author Kunio Hirata. “The manuscript [on our results] is the evidence.”
The resulting structure revealed the path through which positively charged ions are transferred across the cell membrane, resolving an ongoing debate among molecular biologists. A large outer ‘vestibule’ structure at the cellular exterior gives way to a pore lined with negatively charged surfaces, which favor the entry of positively charged ions. This pore is blocked when ChR is inactive, but illumination at the proper wavelength triggers a series of proton transfer events within the protein that eliminate these obstructions, enabling ions to pass. A series of mutation experiments provided additional support for this mechanism.
“[Further] detailed structural information around this pathway should provide useful insights for the precise and principled design of ChR variants with altered ion selectivity and absorption spectra,” says Kato. He and his colleagues now plan to pursue such targeted protein engineering efforts, while also working to obtain additional ChR structures that provide further confirmation for their functional model.
References
- 1. Kato, H.E., Zhang, F., Yizhar, O., Ramakrishnan, C., Nishizawa, T., Hirata, K., Ito, J., Aita, Y., Tsukazaki, T., Hayashi, S. et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482, 369–374 (2012). doi: 10.1038/nature10870
