Jul. 6, 2012 Research Highlight Chemistry
Shining lights of chemistry
Molecules with rare and valuable light-absorbing abilities finally give up their structural secrets
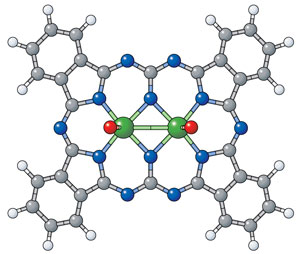 Figure 1: An image of an expanded phthalocyanine reveals the origin of its unusual optical properties: two central metal atoms (green) rather than the usual one (white, hydrogen; grey, carbon; blue, nitrogen; red, oxygen; green, molybdenum). © 2012 Atsuya Muranaka, RIKEN Advanced Science Institute
Figure 1: An image of an expanded phthalocyanine reveals the origin of its unusual optical properties: two central metal atoms (green) rather than the usual one (white, hydrogen; grey, carbon; blue, nitrogen; red, oxygen; green, molybdenum). © 2012 Atsuya Muranaka, RIKEN Advanced Science Institute
Potential applications for organic molecules that can interact with light in unusual ways range from next-generation solar cells to light-activated anticancer drugs. One of the oldest families of these brightly colored molecules is the phthalocyanines that were discovered in 1907 and subsequently prized by industry as dyes. A research team from Japan and Russia recently discovered a new branch of this family1. The unusual light-capturing properties of these so-called ‘expanded phthalocyanines’ look set to springboard the phthalocyanines into a range of high-tech applications.
Nagao Kobayashi at Tohoku University in Japan, and Evgeny Luk’yanets at the Organic Intermediates and Dyes Institute in Russia, led the research. “They were discovered accidentally,” says Atsuya Muranaka, a team member based at Japan’s RIKEN Advanced Science Institute in Wako. “It took about 10 years for us to determine their exact molecular structure.”
Phthalocyanines are flat, disc-like molecules with a hole at the center, within which usually sits a single atom of copper or a similar metal, forming brightly colored compounds ranging from blue to green. But the researchers discovered that when they made phthalocyanines from certain salts of the metals molybdenum or tungsten, a small amount of brown-colored material could also form. Muranaka and his colleagues have now run the full gamut of analytical tests, including x-ray analysis, to reveal the compounds responsible. They are indeed expanded phthalocyanines, containing not one but two metal atoms within their central cavity (Fig. 1).
Thanks to their stretched structure, the expanded phthalocyanines have different properties—especially their optical properties—compared with regular phthalocyanines, Muranaka says. “The expanded phthalocyanines absorb near-infrared light, in a range where few other organic molecules [can],” he says.
One potential application for the unusual light-capturing ability of the expanded phthalocyanines could be within tandem solar cells, in which two active compounds are used to gather more solar rays than a single compound can capture. It could also be useful in a cancer treatment called photodynamic therapy, in which light-absorbing compounds are used to generate a form of oxygen known as ‘singlet oxygen’ that kills cancer cells. By carefully aiming light at the tumor, only the cancerous cells are killed by these molecules. As infrared light passes particularly well through human tissue, photodynamic compounds that absorb this light are particularly sought after.
Before they consider such applications, however, Muranaka and his colleagues first plan to find better ways to make expanded phthalocyanines. “At present, they are obtained in quite low yield,” he says.
References
- 1. Matsushita, O., Derkacheva, V.M., Muranaka, A., Shimizu, S., Uchiyama, M., Luk’yanets, E.A. & Kobayashi, N. Journal of the American Chemical Society 134, 3411–3418 (2012). doi: 10.1021/ja209589x
