Aug. 10, 2012 Research Highlight Biology
A new starring role for astrocytes
Identification of a novel membrane barrier in astrocytes may illuminate how neurological signaling is disrupted in patients with Alzheimer’s and epilepsy
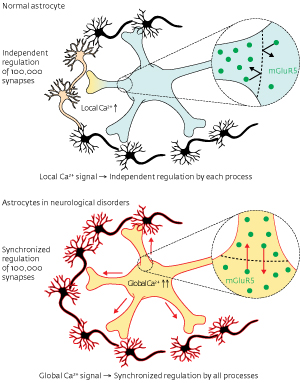 Figure 1: Healthy astrocytes send separate signals through each process, while astrocytes in neurological disorders send synchronous signals to their entire network. © 2012 Katsuhiko Mikoshiba, Misa Arizono and Hiroko Bannai, RIKEN Brain Science Institute
Figure 1: Healthy astrocytes send separate signals through each process, while astrocytes in neurological disorders send synchronous signals to their entire network. © 2012 Katsuhiko Mikoshiba, Misa Arizono and Hiroko Bannai, RIKEN Brain Science Institute
Astrocytes, previously thought of as helper cells for neurons, have recently been shown to send signals themselves. The signals are chemical not electrical and astrocytes send them to neurons, vascular cells and other astrocytes to improve the efficiency of synaptic signaling. A team led by Katsuhiko Mikoshiba and Hiroko Bannai at the RIKEN Brain Science Institute, Wako, have described the mechanism that allows astrocytes to signal each cell in their network individually1.
Named for their star-like shape, astrocytes have a central ‘soma’ and many ray-like arms connecting to the cells they regulate. Healthy astrocytes send separate Ca2+ signals through each ray, called a ‘process’. Signaling was known to be regulated by a receptor in the cellular membrane called the metabotropic glutamate receptor (mGluR5), but it was unclear how the signals were confined to individual processes. Understanding this specificity may be therapeutically important because in brains affected with Alzheimer’s disease or epilepsy astrocytes send global signals, more like a megaphone broadcast than the telephone calls made by healthy astrocytes (Fig. 1).
To understand how astrocyte signaling is regulated, the researchers tagged individual mGluR5 receptors with quantum dots—semiconductor nano-crystals that emit light when excited—then observed how the receptors migrated through the fluid membrane. Video footage revealed that receptors did not pass from the process to the soma. In normal astrocytes, the mGluR5-selective diffusion barrier could, by compartmentalization of Ca2+ signaling, allow each process to regulate its contacting partners independently.
To investigate the character of the barrier, Mikoshiba’s team attempted to undermine it. Over-expression of mGluR5 overwhelmed the barrier, which they infer is made of proteins that interact with the cytosolic portion of mGluR5. Each barrier protein pairs with a single mGluR5 molecule and prevents it from crossing to the soma. However, the number of barrier proteins is finite and an overabundance of mGluR5 leaves some receptors free to cross into the soma, thus enabling propagation of global signals through every process in the astrocyte.
Experimental models of Alzheimer’s disease and epilepsy have shown increased concentrations of mGlu5 in astrocytes. The researchers believe that understanding the molecular nature of the diffusion barrier will provide new targets for the treatment of these conditions. Once they reveal the molecular nature of the barrier, the team hopes to produce a transgenic mouse lacking the astrocytic barrier protein. “We are very curious to know the effect of global astrocytic Ca2+ signaling on the neuronal network and neuro-vascular coupling,” says Mikoshiba.
References
- 1. Arizono, M., Bannai, H., Nakamura, K., Niwa, F., Enomoto, M., Matsu-ura, T., Miyamoto, A., Sherwood, M.W., Nakamura, T. & Mikoshiba, K. Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation. Science Signaling 5, ra27 (2012). doi: 10.1126/scisignal.2002498
