Sep. 28, 2012 Research Highlight Biology
Combating cellular heat stress
When the body’s exposed to heat shock, a recently identified ‘firefighter’ molecule transports heat-response proteins into the cell nucleus to prevent damage
 Figure 1: In response to heat shock, such as a steam burn to the forearm, cells respond to protect nuclear proteins from damage. © iStockphoto.com/tirc83
Figure 1: In response to heat shock, such as a steam burn to the forearm, cells respond to protect nuclear proteins from damage. © iStockphoto.com/tirc83
Akin to sending firefighters in response to a house fire, cells deploy a brigade of heat shock proteins (HSPs) when exposed to environmental stresses, such as a rapid shift in temperature, or ‘heat shock’ (Fig. 1). Heat shock is a stressor that perturbs a cell’s equilibrium, or homeostasis, by causing proteins to misfold and aggregate. HSPs are molecular chaperones that are essential for maintaining this homeostasis. In response to heat shock and other cellular stresses, HSP expression increases and these proteins are transported into the nucleus to protect cells from stress-induced misfolding.
Cell biologists think that the trafficking of most proteins into and out of the nucleus is controlled by the importin-β family proteins. However, the activity of the protein RanGTP, which provides the energy for importin-β-mediated nuclear trafficking, is reduced under heat shock stress conditions. Thus, the molecular mechanism facilitating the transport of HSPs into the nucleus has remained unclear.
Now, a team of cell biologists led by Naoko Imamoto at the RIKEN Advanced Science Institute, Wako, has solved this apparent paradox. In a study published recently in the journal Cell1, Imamoto and her colleagues have identified a previously unknown nuclear import pathway that is triggered by heat-shock stress. Further, the pathway includes a hitherto unknown protein that binds to HSPs and carries them into the nucleus through pores in the nuclear membrane.
Understanding transport under heat stress
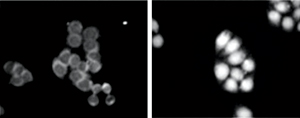 Figure 2: Fluorescently labeled Hsc70 localizes to the nucleus in reconstituted preparations from heat-shocked cells (right) but not normal cells (left). © 2012 Naoko Imamoto, RIKEN Advanced Science Institute
Figure 2: Fluorescently labeled Hsc70 localizes to the nucleus in reconstituted preparations from heat-shocked cells (right) but not normal cells (left). © 2012 Naoko Imamoto, RIKEN Advanced Science Institute
In one series of experiments, the researchers reconstituted the nuclear trafficking of HSPs in Petri dishes. Initially, they removed the fluid from normal and heat-shocked HeLa cells, which were originally derived from a human cervical tumor. They used it to make four different preparations, in which fluid taken from normal or heat-shocked cells was added to normal cells or those treated with heat shock, and then incubated with a fluorescently labeled HSP called Hsc70.
This revealed that Hsc70 is only transported into the nucleus in those preparations containing fluid removed from heat shock-treated cells. This occurred only when ATP, a ubiquitous molecule used by all cells as an energy source, was added to the Petri dishes (Fig. 2).
Proteins migrate into the nucleus through a protein assembly called the nuclear pore complex (NPC), which spans the membrane of the nucleus. NPCs are enormous structures consisting of some 30 different molecules called nucleoporins. The process of transporting proteins through NPCs involves several stages. First, the ‘cargo’ protein set for transportation binds to a carrier molecule. The carrier translocates through NPCs by interacting with the nucleoporins, and releases its cargo once inside.
“Nuclear transport is my main research topic,” says Imamoto. “We identified importin-β and importin-α in 1995. The precise mechanism of how these carrier proteins translocate through the nuclear pore complexes (NPCs) is still unclear, but it is widely accepted to occur through interactions between the carriers and water-repellant regions of NPC components called nucleoporins.”
Carrier proteins, such as importins, are strongly repelled by water. Imamoto and her colleagues exploited this fact to isolate the carrier that binds to Hsc70. They again removed the fluid from heat-shocked cells, and then ran it over a water-repellent column containing protein-binding beads. In this way, they identified a small protein that is encoded by a gene on chromosome 11, and named it Hikeshi, after the firefighters known as hikeshi during the Edo period in Japan.
“Nuclear transport is key for regulating cellular function, and there are diverse transport pathways mediated by different importin family in cells. We wondered whether different transport pathways function under different cellular conditions,” Imamoto explains. “Nobody expected that a completely different nuclear transport pathway would exist in cells.”
Further investigation revealed that: expression levels of the Hikeshi protein increase by 2- to 3-fold in response to heat shock; Hikeshi both binds to Hsc70 and interacts with nucleoporins; and binding of Hikeshi to Hsc70 is regulated by co-chaperones. Hikeshi binds only to Hsc70 molecules that have ATP attached to them, and the proteins dissociate from each other when ATP is broken down to ADP, a reaction that releases energy.
Based on this evidence, Imamoto and her colleagues propose that the energy needed for Hikeshi-mediated transport could come from the ATP–ADP cycle of Hsp70s, instead of that of RanGTP, as in the case of importin β family-mediated transport. The researchers also found that Hikeshi is sufficient and essential for the nuclear transport of Hsc70, and that it reverses the damage that heat shock causes to the nucleus. In fact, cells cannot survive heat shock treatment without it.
Solving one mystery reveals another
Imamoto and her team’s findings show that cells activate distinct nuclear transport pathways under normal and heat shock stress conditions. When normal conditions prevail, the importin-β molecules mediate the transport of proteins into and out of the nucleus. This pathway is deactivated by heat shock stress, during which cells switch to the Hikeshi pathway, so that Hsc70 can be accumulated into the nucleus to counteract the cellular damages induced by heat-shock stress.
Exactly how cells switch from one nuclear transport pathway to the other, however, is not clear. It is possible that Hikeshi carries cargoes other than Hsc70 into the nucleus during heat shock stress conditions.
“Further study of the switching mechanism should provide new insights into the regulation of both the nuclear transport system and the molecular chaperone system,” says Imamoto. “Another intriguing question is the molecular mechanism underlying activation of Hikeshi-mediated nuclear transport.”
References
- 1. Kose, S., Furuta, M. & Imamoto, N. Hikeshi, a nuclear import carrier for Hsp70s, protects cells from heat shock-induced nuclear damage. Cell 149, 578–589 (2012). doi: 10.1016/j.cell.2012.02.058
About the Researcher
Naoko Imamoto

Naoko Imamoto was born in Osaka, Japan. She received her Bachelor's degree in Biology from the Osaka University Faculty of Science, her Master’s degree in Medical Science from Osaka University, and her PhD from Osaka University Medical School. Part of her initial work was the identification of importin α and importin β as nuclear transport receptors and carriers in 1995. In 2000, she became independent as an associate professor at the National Institute of Genetics. In 2002 Imamoto took up the position of chief scientist and established the Cellular Dynamics Laboratory at RIKEN. Her current research areas involve the mechanism and regulation of nuclear transport and the biogenesis of the nuclear pore complex. Nuclear transport affects various cellular processes, while nuclear pore complex biogenesis involves various cellular processes such as cell cycle, nuclear envelope, membrane lipid and chromatin. Imamoto aims to develop fusion domains as a new area of research.
