Nov. 2, 2012 Research Highlight Biology
First mouse, now human, lab-grown eye tissue
Human embryonic stem cells can self-organize into eye-like structures via a cell-culture technique developed at RIKEN
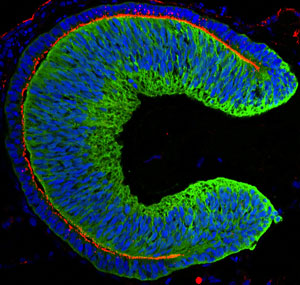 Figure 1: An embryonic eye derived from human embryonic stem cells. © 2012 Elsevier
Figure 1: An embryonic eye derived from human embryonic stem cells. © 2012 Elsevier
Producing retinal tissue from human embryonic stem cells is now possible thanks to a team of researchers led by Yoshiki Sasai of the RIKEN Center for Developmental Biology in Kobe1.
Sasai and his colleagues have developed a novel cell culture method in which embryonic stem (ES) cells are grown in suspension instead of on a flat surface. ES cells grown under these conditions can organize themselves into complex three-dimensional structures when they are treated with the appropriate combination of growth factors.
Last year, Sasai’s team reported that mouse ES cells cultured in this way recapitulate developmental mechanisms and self-organize into a cupped, layered structure that resembles the embryonic eye and contains all the cell types found in the mature retina, including photoreceptor cells2.
In their latest study, the team repeated these experiments using human ES cells, and found major differences in how they form eye-like structures. The structures derived from human ES cells were substantially larger and thicker than those formed by mouse cells, reflecting the differences in size between the two species (Fig. 1). And unlike the structures formed from mouse cells, the human-based structures also had a tendency to curve more at the edges.
Importantly, the human ES cells took significantly longer to form embryonic eyes—more than 100 days compared to just 20 days for mouse cells, presumably reflecting the differences in normal gestation times. This made the experiments technically challenging, because it is difficult to maintain stable cell cultures for periods of longer several weeks.
Sasai and his colleagues noticed, however, that the cell cultures that grew well during the first month tended to generate well-formed retinal tissue. To keep the cultures stable at this critical stage, they developed a novel cryonic preservation method for storing the tissue at this critical intermediate stage.
The cryopreservation method involves cutting the retinal tissue from the cupped structures after 18 days in culture and then leaving it to continue growing in suspension for another 12 days. The tissue is then briefly cooled on ice before being submerged in liquid nitrogen. Crucially, the tissue can be stored in this state for long periods of time, but remains healthy and continues to grow when thawed later on.
“We now plan to test the functionality by grafting these tissues into animal eyes,” says Sasai. “The most straightforward application would be for transplantation to patients suffering from retinitis pigmentosa, in which photoreceptors gradually degenerate, leading to blindness.”
References
- 1. Nakano, T., Ando, S., Takata, N., Kawada, M., Muguruma, K., Sekiguchi, K., Saito, K., Yonemura, S., Eiraku, M. & Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012). doi: 10.1016/j.stem.2012.05.009
- 2. Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T. & Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011). doi: 10.1038/nature09941
