May 23, 2014 Research Highlight Biology
A sensitive cog in the mammalian clock
A missing piece of the mammalian circadian machinery may modulate the body’s internal sense of time in response to stress
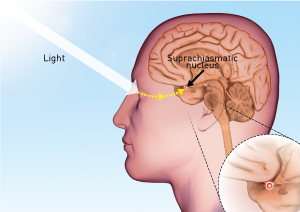 Figure 1: The mammalian circadian rhythm is set by the suprachiasmatic nucleus in response to visual light and dark stimuli. © 2014 RIKEN (foreground); © Dorling Kindersley/Thinkstock (background)
Figure 1: The mammalian circadian rhythm is set by the suprachiasmatic nucleus in response to visual light and dark stimuli. © 2014 RIKEN (foreground); © Dorling Kindersley/Thinkstock (background)
Most organisms have a suite of ‘clock’ genes that establish their internal circadian rhythm of day and night in response to the rising and setting of the Sun. Scientists have mapped the core components of the circadian machinery in mammals, but the full extent of clock genes remains unknown. Research led by Toru Takumi and colleagues from the RIKEN Brain Science Institute has now revealed a new component of the mammalian clock that exhibits some intriguing properties relative to the other circadian genes1.
A key brain region involved in the mammalian clock is the suprachiasmatic nucleus (SCN) within the hypothalamus (Fig. 1). The SCN decodes the light and dark signals generated by our eyes, and responds by turning certain clock genes on or off and relaying these circadian rhythms throughout the body. Associated with the action of the SCN is the protein BMAL1, which controls the rise and fall of clock gene activity. In research aimed at identifying new genes associated with BMAL1, Takumi and his colleagues undertook a genome-wide screen to search for targets regulated by this protein.
The search uncovered a mouse gene called chromatin immunoprecipitation (ChIP)-derived repressor of network oscillator (Chrono). The levels of Chrono protein oscillate in the SCN and various other organs in coordination with BMAL1. BMAL1 binds directly to the Chrono gene and controls the production of the Chrono protein; Chrono in turn binds to other known clock proteins to inhibit activity.
Chrono inhibits many genes activated by BMAL1 in a manner similar to two other circadian proteins, cryptochrome (Cry) 1 and 2. These three proteins exert parallel but seemingly independent effects. Mice lacking Chrono but retaining Cry1 and Cry2 show skewed but functional circadian rhythms, and computational models suggest that loss of all three may be necessary to abolish these rhythms entirely. “One of our goals is to make triple-knockout mice of Chrono, Cry1 and Cry2 and see whether they become completely arrhythmic,” says Takumi.
The hypothalamus governs numerous critical physiological functions, and the researchers uncovered intriguing evidence that Chrono activity in the SCN is modulated by hormones associated with the brain’s response to stress. “This is not seen in other negative elements like Cry2, so Chrono might link circadian rhythm with higher cognitive functions like mood,” says Takumi. He notes that unlike virtually every other component of the cellular clock, Chrono appears to be unique to mammals. “It may therefore have a specific contribution in maintaining circadian rhythms in higher organisms,” says Takumi.
References
- 1. Goriki, A., Hatanaka, F., Myung, J., Kim, J. K., Yoritaka, T., Tanoue, S., Abe, T., Kiyonari, H., Fujimoto, K., Kato, Y. et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biology 12, e1001839 (2014). doi: 10.1371/journal.pbio.1001839
