Dec. 4, 2015 Research Highlight Chemistry
Building better bilayers
A strategy for generating stable lipid bilayers could simplify the study of biologically important membrane proteins
An approach for constructing artificial membranes that promises to make proteins in cell membranes easier to study has been developed by a team of RIKEN researchers1.
Roughly 25 to 30 per cent of all human proteins reside in cellular membranes, where they help cells respond to changing conditions by relaying information from the outside environment to the interior of the cell. These membrane proteins are critical targets for treating diseases.
It is vital to know the structures of proteins when exploring their biological functions. The structures of membrane proteins can be deduced by embedding them in double-layer slabs of phospholipids known as bicelles, which mimic sections of the cell membrane. These bicelles are then analyzed using a technique called nuclear magnetic resonance spectroscopy. In this technique, the proteins under investigation are exposed to a magnetic field that causes them to spontaneously align, enabling detailed structural analysis.
However, generating robust bicelles is problematic. “Conventional bicelles only attain optimal alignment under limited conditions in terms of temperature, pH and so on,” says Yasuhiro Ishida of the RIKEN Center for Emergent Matter Science, who led the study. He and his team set out to design a simple strategy for building highly stable bicelles.
Stabilization is necessary since one end of a phospholipid preferentially interacts with water, whereas its other end is repelled by water, resulting in the formation of sandwich-like bilayers. Chemicals known as surfactants are generally used to cap the rims of the bicelles.
Ishida’s team improved on this approach by using special surfactants that can assemble themselves into multimolecular polymers (Fig. 1). These surfactants bind much more tightly to the bilayer rims than individual surfactant molecules. Consequently, the resulting bicelles exhibited remarkable stability and a capacity for robust magnetically induced alignment over a wide temperature range―from room temperature to more than 90 degrees Celsius.
“Other research groups have taken rather complicated approaches where the stabilization effects are not satisfactory,” says Ishida. “This work demonstrates that a very simple approach based on polymerization works highly efficiently.”
This remarkable temperature stability means that these bicelles could be used to study membrane proteins that are normally active at high temperatures. The researchers are now working to refine their approach so that it performs equally well at near-freezing temperatures.
Ishida also sees potential applications for experimentally modeling cellular processes. “Our bicelle systems could be useful as a simple and easy-to-handle model of biological membranes,” he says.
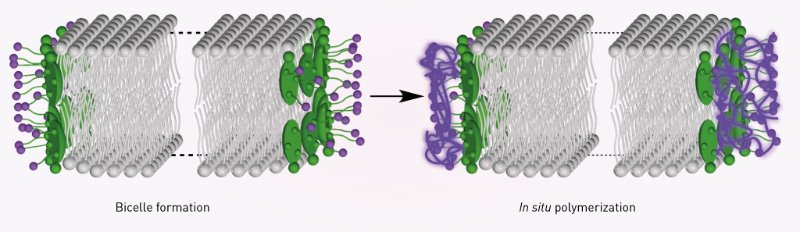 Figure 1: The formation of bicelles (left) is stabilized by polymerization (right) of the surfactant molecules (green) that flank the phospholipid bilayer (gray). © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Figure 1: The formation of bicelles (left) is stabilized by polymerization (right) of the surfactant molecules (green) that flank the phospholipid bilayer (gray). © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
References
- 1. Matsui, R., Ohtani, M., Yamada, K., Hikima, T., Takata, M., Nakamura, T., Koshino, H., Ishida, Y. & Aida, T. Chemically locked bicelles with high thermal and kinetic stability. Angewandte Chemie International Edition 54, 13284–13288 (2015). doi: 10.1002/anie.201506781
