May 29, 2015 Perspectives Medicine / Disease
Health, wealth and longevity
To achieve a holistic approach to medicine that balances longevity with quality of life, RIKEN researchers are working to allow the diagnosis and treatment of diseases with unprecedented precision.
 © 2015 RIKEN
© 2015 RIKEN
The phrase ‘the Japan syndrome’ has often been used to describe socioeconomic upheavals unique to the island state, such as nuclear disasters, financial crises and reliance on grain imports. Recently, it has come to refer to the dramatic demographic changes Japan is facing and which also threaten nations globally.
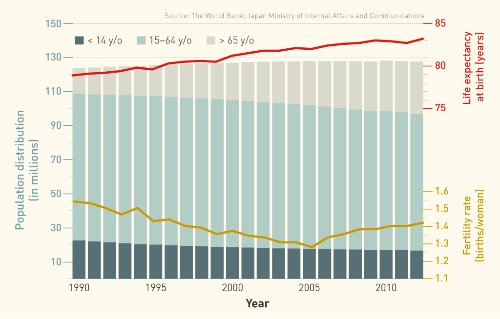
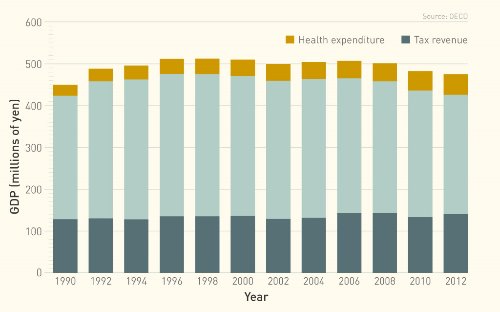
Japan tops the list of the most aged countries in the world, with more than 30 per cent of its population over the age of 60 and an average life expectancy of almost 84. Combined with a low birth rate, this high life expectancy has seen the workforce shrink, slashing tax revenues needed to pay for ballooning social security expenses. In Japan, there are now only two working-age adults per elderly person, down from nearly six in 1990. And spending on medical care rose by almost USD 171 billion between 1995 and 2013, reaching 10.3 per cent of the gross domestic product (GDP).
Improved medical care offers many opportunities for alleviating the burden of this vicious demographic cycle by extending the number of active and healthy years available to an exploding elderly population. In this way, societies can benefit from increased productivity and economic growth. The RIKEN Preventive Medicine & Diagnosis Innovation Program (PMI) is driving advances in treatment and diagnosis by bringing the results of research at RIKEN in biology, physics, chemistry and engineering to the clinic.
Preemptive seven
 © 2015 RIKEN
© 2015 RIKEN
Elias Zerhouni, former director of the US National Institutes of Health, first emphasized the necessity of shifting from curative to preventive medicine to maximize wellness at a national level. He argued for the need to manage the entire life cycle of a disease, from susceptibility and risk, to prevention, diagnosis, treatment and cure. Zerhouni identified four long-term goals, branded “the 4 ‘P’s of medicine”, of making medical care more ‘predictive’, ‘personalized’, ‘preventive’ and ‘participatory’.
Recent large-scale studies of the genome and its many ‘ome’ derivatives, such as proteomics and metabolomics, together with other breakthrough technologies, have opened the possibility of adding three more ‘P’s—we can now strive to deliver ‘precise’ and ‘preemptive’ medicine at the ‘point of care’. When executed together, these seven ambitious goals will lead to diagnoses and treatments that account for the unique biological characteristics of individual patients as well as those of different types of diseases. It will, for example, catch emerging malignancies earlier than before and consider inherited variations when prescribing drugs.
Precision medicine will intervene at the right time and place, taking snapshots of a patient’s health that reflect their present state. Take influenza, for example, a common viral infection typically left to run its course of sniffles, fever and misery, which can rapidly deteriorate into more serious lung and brain infections. Dangerous complications can be prevented by precisely identifying the causative virus at the point of care.
In 2009, a virulent strain of the influenza virus A (H1N1), or swine flu, spread across Japan and the world, causing about 18,000 deaths. While diagnostic tests were used to detect the virus, they proved unreliable during the early stages of infection. In 2012, researchers at the RIKEN Omics Science Center, the PMI’s predecessor organization, developed the RT-SmartAmp assay, a simple, quick and sensitive method for clinically detecting swine flu within 24 hours of fever onset1. The technique involves reverse transcribing a specific sequence of viral RNA into DNA and isothermally amplifying the complementary DNA strands—all in one reaction tube, in contrast to the two-step process of conventional polymerase chain reaction amplification. The technique could be applied to various other infectious diseases.
Omics prognostics
Immediate and accurate detection and diagnosis of many more diseases will soon be possible as omics research advances, allowing practitioners to spot unusual developments before they mature into debilitating diseases, and administer targeted treatments.
Sequencing the entire human genome has provided researchers with tremendous information about the genetic basis of disease. The field of genomics is saturated with scientists searching for correlations between specific diseases and germline mutations, which are passed down from generation to generation. Some genetic errors are associated with serious disorders, like the single-nucleotide swap in the β-globin gene that causes the production of sickle-shaped red blood cells. Other mutations increase the risk of acquiring a disease, such as the abnormal BRAC1 gene that makes actress Angelina Jolie and thousands of others more susceptible to breast and ovarian cancers.
While genomics can provide important information about an individual’s risk of succumbing to a disease, it offers only a static profile. Cells are teeming with activity. The expression of genes is closely regulated by short nucleic acid sequences and proteins to ensure proper cell development, differentiation and homeostasis. These regulatory mechanisms can vary between the nearly 400 distinct cell types in the body.
Scientists are only just beginning to identify the many elements of this regulatory process, which include promoter and enhancer sequences of DNA, enhancer RNA, messenger RNA, microRNA sequences and long non-coding RNA that facilitate protein translation. These discoveries have opened up new areas of research.
FANTOM5’s interdisciplinary approach
 FANTOM5 members at a meeting in the RIKEN Yokohama campus
FANTOM5 members at a meeting in the RIKEN Yokohama campus
PMI researchers are leading these efforts through their contributions to the research consortium established in 2000, named Functional Annotation of the Mammalian Genome (FANTOM).
FANTOM takes a multi-omics approach, integrating large-scale analyses of the genome with maps of transcripts, transcription factor proteins, promoters2 and enhancers3 to develop new biomarkers of disease. The fifth edition, FANTOM5, now provides proteome, transcriptome, promotome and enhancerome analyses for 180 mammalian cell type.
In one of many potential applications of these cell-specific expression profiles, a team of researchers, including Masayoshi Itoh and Hideya Kawaji affiliated with the PMI, are developing biomarkers to specifically tag corneal endothelial cells derived from induced pluripotent stem cells4. Corneal endothelium is crucial for maintaining transparency in the eye’s outer layer. The technique could be used in the regeneration and surgical transplantation of corneal endothelium into patients with damaged corneal tissue due to injury or inherited degenerative diseases such as Fuchs’ Dystrophy.
Recently, the FANTOM5 consortium and an international team of researchers, including Itoh, Kawaji and Jun Kawai at the PMI, identified associations between common diseases and single-nucleotide errors in non-protein-coding enhancer regions—mutations previously overlooked by researchers focusing primarily on the protein-coding segments of DNA. They also found a disproportionately high number of mutations in enhancers predominantly expressed in pathologically relevant cell types. The researchers were then able to link abnormal enhancer activity to disorders primarily found in that type of cell, such as Grave’s disease and thyroid tissue, or chronic lymphocytic leukemia and lymphocytes.
In a separate study on transcribed enhancer sequences known as enhancer RNA, FANTOM5 researchers concluded that enhancer RNA molecules are the first to become active in a wave of transcriptional activity that occurs when cells differentiate into more specialized states5.
A crucial factor of FANTOM’s success has been its interdisciplinary approach. The consortium brings together more than 500 researchers from 20 countries with expertise in areas as far-reaching as molecular biology, bioinformatics and engineering. The PMI is replicating this strategy to introduce precision technologies enhanced by omics to research laboratories and hospitals.
From lab to patient
As Japan’s largest research institute, RIKEN is uniquely positioned to address the multifaceted needs of researchers, clinicians and the medical industry.
To identify linkages across disciplines and encourage the sharing of ideas and learning across the institute, the PMI conducted an institution-wide survey of 400 research projects and 39 principal investigators. The initiative led to 90 new projects and two PMI research units embedded in the RIKEN Center for Life Science Technologies and the RIKEN Advanced Center for Computing and Communication.
After identifying the critical needs of research, the PMI has gone on to investigate the demands of practice by conducting hundreds of in-depth interviews with clinicians at Juntendo University Hospital and several other universities in Japan. Doctors were asked to reflect on the seven ‘P’s and to specify the kinds of information, tools and equipment that could improve diagnostics, therapeutics and data management in their fields. The PMI is now mining these statements to develop innovative research projects at RIKEN, which could lead to some of the safest hospitals in the world.
With sharper physiological profiling, earlier disease recognition, targeted treatment and faster recovery, people in rapidly aging societies will enjoy longer, healthier and more productive lives.
References
- 1. Kawai, Y., Kimura, Y., Lezhava, A., Kanamori, H., Usui, K., Hanami, T., Soma, T., Morlighem, J. É., Saga, S., Ishizu, Y. et al. One-step detection of the 2009 pandemic influenza A(H1N1) virus by the RT-SmartAmp assay and its clinical validation. PLoS ONE 7, e30236 (2012). doi: 10.1371/journal.pone.0030236
- 2. The FANTOM Consortium and the RIKEN PMI and CLST (DGT). A promoter-level mammalian expression atlas. Nature 507, 462–470 (2014). doi: 10.1038/nature13182
- 3. Andersson, R., Gebhard, C., Miguel-Escalada, I., Hoof, I., Bornholdt, J., Boyd, M., Chen, Y., Zhao, X., Schmidl, C., Suzuki, T. et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 (2014). doi: 10.1038/nature12787
- 4. Yoshihara, M., Ohmiya, H., Hara, S., Kawasaki, S., FANTOM consortium, Hayashizaki, Y., Itoh, M., Kawaji, H., Tsujikawa, M. & Nishida, K. Discovery of molecular markers to discriminate corneal endothelial cells in the human body. PLoS ONE 10, e0117581 (2015). doi: 10.1371/journal.pone.0117581
- 5. Arner, E., Daub, C. O., Vitting-Seerup, K., Andersson, R., Lilje, B., Drabløs, F., Lennartsson, A., Rönnerblad, M., Hrydziuszko, O., Vitezic, M. et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 347, 1010–1014 (2015). doi: 10.1126/science.1259418
About the Researcher
Yoshihide Hayashizaki

Yoshihide Hayashizaki has been director of the RIKEN Preventive Medicine & Diagnosis Innovation Program since it was set up in 2013. He established the international research consortium Functional Annotation of Mammalian Genome (FANTOM) in 2000, building on his initial work developing technologies to produce full-length cDNA libraries.
