Sep. 2, 2016 Perspectives Biology
Controlling the dynamics of life
The molecular biology, researchers at RIKEN are pursuing the promise of unprecedented control over health and disease with the emergence of a truly interdisciplinary field of study.
 A truly interdisciplinary field of biology is bringing together mathematics and experimental results to gain unprecedented control over health and disease. © a-r-t-i-s-t/DigitalVision Vectors/Getty
A truly interdisciplinary field of biology is bringing together mathematics and experimental results to gain unprecedented control over health and disease. © a-r-t-i-s-t/DigitalVision Vectors/Getty
Biologists and physicists are often seen as being at opposite ends of science. While both attempt to explain and predict the world around us using the language of science, the dialect of physics and mathematics is not easy to translate into the dialect of biology.
In the field of biology, these two dialects are converging in a way never seen before. The techniques and approaches used by these seemingly disparate branches of science are being exchanged to yield new and far-reaching benefits.
On one hand are the reductionists, who try to explain complex systems by breaking them down into simpler component parts. With access to a vast repertoire of microscopic experimentation and observation, these keen-eyed examiners try to implicate faulty genes and mutant proteins in diseases, or infer details about living organisms from cells isolated in Petri dishes.
On the other hand are the theorists, who try to establish fundamental principles in the biological universe. A single-nucleotide shift, they argue, cannot possibly explain the behavior of an entire organism. Instead, the many molecular components continuously interact to form an integrated, robust and dynamic network that expresses a wide range of biological states.
Both approaches are limited in their ability to describe biological processes. The former stops time and confines space to take a closer look, while the latter listens for faint harmonies in a cacophony of noise. An integrated approach is now taking hold of the field, with researchers at RIKEN at the forefront of taking control of health to sideline disease.
Parts of a puzzle
 Massive catalogues of the genes, RNA transcripts, epigenetic modifications, proteins and metabolites in living organisms offer a static picture of the complex web of molecular structures. © liuzishan/iStock/Getty Images Plus
Massive catalogues of the genes, RNA transcripts, epigenetic modifications, proteins and metabolites in living organisms offer a static picture of the complex web of molecular structures. © liuzishan/iStock/Getty Images Plus
The origins of modern molecular biology are often traced back to a series of studies in the mid-twentieth century in which scientists first isolated DNA, determined its chemical components, and revealed its three-dimensional double-helix structure. These breakthroughs were followed with the discovery of restriction enzymes that could cleave DNA at specific sites and detach short manageable fragments from the tangled string of the larger molecule. Technologies emerged, such as polymerase chain reaction (PCR), to clone millions of copies of these DNA fragments for closer analysis.
The 1970s saw the arrival of simple and quick methods for sequencing purified strands, culminating in the 2000s with the completion of the Human Genome Project to spell out all 3 billion letters in the multi-volume book that is the human genome. Further automation and refinement of sequencing techniques have dramatically sped up and reduced the cost of gene sequencing.
These advances have enabled the careful and controlled study of individual genes. By editing, cutting and pasting sections of the genetic code, often to generate mutations and model organisms, researchers have been able to link specific genes with specific cellular processes and, ultimately, diseases.
Until recently, well-respected molecular biologists could spend their entire lives studying the biological function of a single gene or molecule. But the same technologies that facilitated such diligent research, have also led to an explosion of information about the rest of the DNA, RNA and proteins swimming in the cellular soup. The genomes of thousands of microbe species have been sequenced, and thousands more of individual specimens within the same species. Added to these complete genomic registers have been the characterization of entire sets of RNA transcripts, epigenetic modifications, proteins and metabolites produced by an organism.
This phenomenal production of information transformed a nascent field of bioinformatics into a booming industry—the ‘omics’ industry—turning raw data into insights. Researchers, especially in the United States, began developing computational techniques and statistical models to annotate and analyze the data using powerful supercomputers. The result is a complex web linking multiple genes to multiple metabolites and multiple products.
But even after overcoming the difficulties of piecing together the billions of puzzle pieces of living organisms, many argue that this approach brings researchers no closer to understanding how the different parts connect over time, giving rise to the dynamics of life. A somewhat older, but less well-known approach, to molecular biology offers an alternative.
Summing up cells
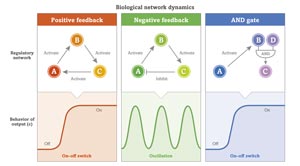 Biological networks are controlled by self-regulating pathways of positive and negative feedback. © 2016 RIKEN
Biological networks are controlled by self-regulating pathways of positive and negative feedback. © 2016 RIKEN
Pen, paper and a clear head established the theoretical foundations for molecular biology, with the application of differential equations, control theory and non-equilibrium thermodynamics to describe moving molecular networks.
Biologists and biophysicists investigated these concepts in the belief that universal rules also applied to living systems. Well-described rules gave researchers control over biological states, which could eventually be used to correct malfunctioning systems and cure human diseases.
Biological processes typically involve a series of chemical reactions, linked together like a chain of dominoes. The dominant view prior to the establishment of these theoretical frameworks was that a single step in the pathway controlled the rate of production and that speeding up or slowing down that one activity could increase or decrease the output. But several researchers in Europe used mathematical techniques to prove that control over the pathway was shared between many different steps.
These techniques have been applied to the study of a wide range of biological systems, from signaling pathways to gene expression. They reveal the robust and adaptive nature of biological networks and explain why only a small number of diseases can be attributed to single genetic mutations. Proponents of this theoretical approach have even found that the same pattern of genetic expression can manifest as more than one physical trait, contradicting the reductionist view that organisms are no more than the sum of their parts.
With the gradual convergence of previously disparate disciplines, mathematicians and physicists have begun to explore new territories. In 2013, RIKEN launched the Interdisciplinary Theoretical Science Research Group (iTHES) to drive theoretical science in fields ranging from sub-nuclear physics to cosmology and biology. And in April 2016, it established the Center for Advanced Integrated Intelligence Research to carry out fundamental studies into informatics. But in the field of molecular biology, the Laboratory for Integrated Cellular Systems at the RIKEN Center for Integrative Medical Sciences, has made remarkable strides in merging the two worlds of theory and experiment.
Chain reaction
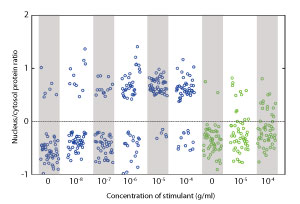 A transcription factor found in B cells is activated and moves from the cytosol to the nucleus in a switch-like manner (blue). Cells in which the mechanism stimulating this switch-like response was deactived, exhibited a more graded response (green). From Ref. 1. Reprinted with permission from AAAS.
A transcription factor found in B cells is activated and moves from the cytosol to the nucleus in a switch-like manner (blue). Cells in which the mechanism stimulating this switch-like response was deactived, exhibited a more graded response (green). From Ref. 1. Reprinted with permission from AAAS.
An interdisciplinary approach to molecular biology involves finding coherent theories to explain the reams of gene and protein expression data acquired from high-throughput experiments. The framework gives meaning to the static networks produced by the ‘omics’ industry.
Biological networks are self-regulating. Steps further along the pathway can control earlier events in one of two ways: positive or negative feedback. Imagine a triangle of three genes: gene A activates gene B, gene B activates gene C, and gene C then activates gene A to create a positive feedback loop. Researchers use mathematical models to explain what effect such regulatory mechanisms, or motifs, might have on cellular dynamics. The activate-activate-activate motif, for example, results in a steep increase in activity, which can set a threshold in the system. But if C instead inhibits A to create a negative feedback loop the result is often a series of oscillations—an important mechanism governing cyclic behavior such as circadian rhythms and cell cycles.
In May 2014, researchers at the RIKEN Center for Integrative Medical Sciences identified an important role of a positive-feedback mechanism in controlling the switch-like response of a transcription factor in a type of white blood cell known as B cells1. These cells defend the body against foreign invaders by producing specialized antibodies that tag pathogens for destruction by other parts of the immune system. Studies that improve our understanding of the underlying mechanisms of B cell activation can help to explain how irregular activity could lead to immune deficiency or lymphoma.
Earlier in May 2010, the RIKEN team, in collaboration with a systems biologist at University College Dublin, found a different regulatory motif to explain the switch-like behavior of a transcription factor in a breast cancer cell line2. The circuit includes an additional molecule, D, and a concept borrowed from control theory: the ‘AND gate’. Both B and D have to activate C to produce the switch-like outcome. The finding also helps to explain how the same pattern of activity can result in different cellular outcomes, depending on the presence or absence of an AND gate.
Interdisciplinary approaches to science require patience and a willingness to approach problems from a different perspective, as the language of physics and mathematics is not easy to translate into the language of biology. Often, such interactions fail to extract simple rules about cellular behavior. But when they do, you can be certain of their far-reaching benefits over time.
References
- 1. Shinohara, H., Behar, M., Inoue, K., Hiroshima, M., Yasuda, T., Nagashima, T., Kimura, S., Sanjo, H., Maeda, S., Yumoto, N. et al. Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-κB activation. Science 344, 760–764 (2014). doi: 10.1126/science.1250020
- 2. Nakakuki, T., Birtwistle, M. R., Saeki, Y., Yumoto, N., Ide, K., Nagashima, T., Brusch, L., Ogunnaike, B. A., Okada-Hatakeyama, M. & Kholodenko, B. N. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell 141, 884–896 (2010). doi: 10.1016/j.cell.2010.03.054
About the Researcher
Mariko Okada

Mariko Okada is head of the Laboratory for Integrated Cellular Systems at the RIKEN Center for Integrative Medical Sciences. Since joining RIKEN in 2000, Okada has been exploring a systems biology approach, combining experiments and computational methodologies to understand cellular regulatory mechanisms. Her current interests are in the mechanisms that induce specific cellular outcomes, including multiscale positive cooperative regulation in signaling pathways and epigenetic regulation.
