May 30, 2008 Research Highlight Chemistry
Working well in a crowded cell
Understanding how glycoproteins are processed in cells may help scientists develop therapies for specific disorders
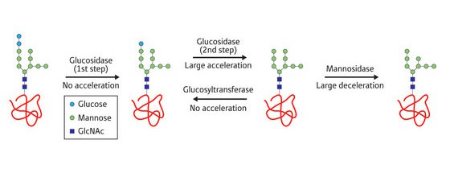 Figure 1: An illustration of the effects of cell-like conditions on glycoprotein processing reactions catalyzed by three enzymes: glucosidase, glucosyltransferase and mannosidase (GlcNAc, N-Acetyl-D-glucosamine). Reproduced in part with permission from Ref.1 © 2008 American Chemical Society
Figure 1: An illustration of the effects of cell-like conditions on glycoprotein processing reactions catalyzed by three enzymes: glucosidase, glucosyltransferase and mannosidase (GlcNAc, N-Acetyl-D-glucosamine). Reproduced in part with permission from Ref.1 © 2008 American Chemical Society
Cells within the body are packed with many different molecules. Many of these molecules play important biological roles. Glycoproteins—proteins that have short sugar chains attached to them—play crucial roles in protein folding, transport and subsequent degradation. Diseases can result when these roles are disrupted; consequently a full appreciation of their function could lead to medical advances.
Now, Yukishige Ito, Kiichiro Totani from the RIKEN Advanced Science Institute (formerly the Discovery Research Institute), Wako, and colleagues have analyzed three key enzymes involved in the processing of glycoproteins under conditions that mimic the crowded environment inside cells1. Although much research has been performed in this area, in most cases, the natural glycoproteins used are not homogenous, says Ito. “In addition, these studies have been conducted in dilute buffer solutions, the environment of which is quite different from that of the inside of living cells.”
The team synthesized a series of glycoprotein sugar chains and studied their reactions as a single entity and bound to additional proteins. Three enzymatic reactions—using glucosidase, glucosyltransferase and mannosidase—were considered under cell-like conditions (Fig. 1). These reactions were compared to similar reactions under standard laboratory dilute conditions.
Surprisingly, the team found that the behavior of certain enzymatic reactions was significantly different under cell-like conditions compared to standard conditions. In particular, when glucosidase was used, the rate at which a specific part of the glycoprotein was trimmed (step 2 of Fig. 1) dramatically accelerated in the cell-like conditions.
When trying to understand how cells work closely matching in-cell conditions is very important. The team achieved this by using various biomacromolecules to crowd the reaction solutions. When they used bovine serum albumin—a protein commonly used in biochemical studies—the trimming rate was greatly enhanced. Similar effects were observed when they used ribonuclease A—another cell enzyme—and high molecular-weight polyethylene glycol. The effects seen are likely the result of conformational changes of the enzymes.
Overall, these latest results of the team have provided a valuable insight into how environment can dramatically alter the way reactions take place and emphasizes the need to mimic real conditions of the cell. Even though this study has taken five years so far, Ito is keen to continue the work. The next step, he says, is to try and prove the biological significance of the current work by studying a wider range of reactions under conditions similar to real cells.
References
- 1. Totani K., Ihara Y., Matsuo I., & Ito Y. Effects of macromolecular crowding on glycoprotein processing enzymes. Journal of the American Chemical Society 130, 2101–2107 (2008). doi: 10.1021/ja077570k
