Nov. 25, 2011 Research Highlight Chemistry
Green fixations for a cleaner future
Copper–carbene catalysts reveal the critical interactions needed to turn waste carbon dioxide into chemical feedstocks
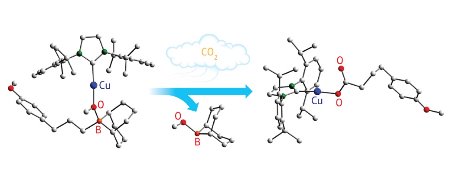 Figure 1: Isolation of a new copper–oxygen–boron complex (left) revealed that carbon dioxide (CO2) can insert into its chemical framework (right) by eliminating the organo-boron component (middle panel, lower structure). © 2011 Zhaomin Hou
Figure 1: Isolation of a new copper–oxygen–boron complex (left) revealed that carbon dioxide (CO2) can insert into its chemical framework (right) by eliminating the organo-boron component (middle panel, lower structure). © 2011 Zhaomin Hou
Using fixation reactions to convert free carbon dioxide (CO2) into different organic molecules is an attractive strategy to cut industrial greenhouse gas levels with marginal waste. Now, broadening the scope of CO2 fixation is possible using a method developed by a research team in Japan led by Zhaomin Hou from the RIKEN Advanced Science Institute in Wako1. The method uses a ‘green’ catalyst system that transforms alkyl–boron molecules into carboxylic acids—an important ingredient for pharmaceutical production.
Organic boron compounds are attractive fixation substrates because they readily participate in carbon–carbon bond-forming reactions. Recently, chemists have used transition metal catalysts to activate hydrocarbons bonded to oxygenated boron esters; addition of CO2 then splits off the activated group and generates a carboxylic acid derivative. However, attempts to reproduce this chemistry with alkylboranes—a widespread class of important synthetic reagents—have had limited success because the so-called ‘catalytic transition metal alkyl’ intermediates are usually unstable and decompose before reacting with CO2.
Hou and colleagues turned to an innovative chemical system to resolve this instability. By combining electron-donating, bulky molecules called N-heterocyclic carbenes (NHCs) with copper atoms, they made metal alkyl complexes that can promote carbon–carbon bond formation with CO2 under mild conditions and at lower cost than most precious metal catalysts—ideal characteristics for sustainably recycling CO2 emissions.
First, the researchers produced an easily activated alkylborane by connecting borabicyclononane (BBN)—a highly strained set of boron–hydrocarbon rings—to the terminal atom of a carbon–carbon double bond. In this approach, the target hydrocarbon for CO2 addition is physically and electronically quite different from the two carbon–boron bonds of the BBN rings.
Hou and colleagues then mixed the alkylborane with the copper–NHC catalyst, a base, and CO2 in a pressurized chamber. After one day at 70 °C, they found that the target had transformed into a new carboxylic acid with near-quantitative yields. Diverse molecules bearing aromatic, halogenated, and bulky functional groups could all act as CO2 fixation substrates using this technique.
The copper–NHC catalyst offered another advantage to the team: a unique chemical environment that enabled isolation of several catalytic intermediates as solid crystals. X-ray measurements of these structures provided the first hard evidence that bonding interactions between alkoxide base molecules, copper atoms, and alkylboranes are critical to enabling CO2 addition (Fig. 1). “Fine-tuning the combination of central metals, bases, and supporting ligands will eventually lead to more efficient and selective catalysts,” notes Hou.
References
- 1. Ohishi, T., Zhang, L., Nishiura, M. & Hou, Z. Carboxylation of alkylboranes byN-heterocyclic carbene copper catalysts: Synthesis of carboxylic acids from terminal alkenes and carbon dioxide.Angewandte Chemie International Edition 50, 8114–8117 (2011). doi: 10.1002/anie.201101769
