Oct. 19, 2012 Research Highlight Chemistry
Water’s triple play at membrane interfaces
Rapid-fire lasers reveal that water molecules adopt three distinct local structures around model lipid monolayers
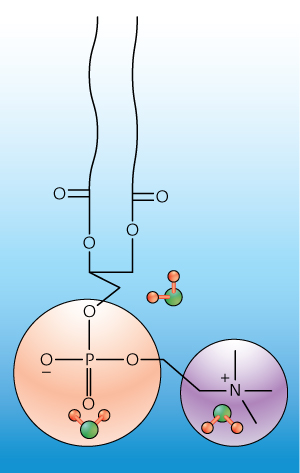 Figure 1: A schematic illustration of three water structures at a model cellular membrane interface: an ‘H-up’ orientation close to a negative phosphate (bottom left), an ‘H-down’ arrangement at a positive amine (bottom right), and a structure in a lipid chain (center). Reproduced, with permission, from Ref. 1 © 2012 American Chemical Society
Figure 1: A schematic illustration of three water structures at a model cellular membrane interface: an ‘H-up’ orientation close to a negative phosphate (bottom left), an ‘H-down’ arrangement at a positive amine (bottom right), and a structure in a lipid chain (center). Reproduced, with permission, from Ref. 1 © 2012 American Chemical Society
Before taking effect, pharmaceuticals that target cell function must cross a crucial barrier: the complex assembly of lipids, proteins, and carbohydrates that make up biological membranes. Recently, scientists realized that besides a membrane’s internal components, thin water layers at its inner and outer surfaces may also play important roles in ion and small molecule transport. However, most characterization methods cannot distinguish between bulk and surface water, so identifying water structures around membranes is challenging.
Tahei Tahara and colleagues from the RIKEN Advanced Science Institute in Wako have overcome this problem by developing a technique that uses ultra-short laser pulses to probe interfacial water1. Using this technique, known as heterodyne-detected vibrational sum frequency generation (HD-VSFG), the team has now discovered that water adopts three structures at a lipid interface containing atoms with both negative and positive charges2, providing critical new evidence of ‘local’ hydration arrangements around different membrane components.
Every human body cell contains a type of lipid called phosphatidylcholines. Their long, saturated hydrocarbon ‘tails’ enable them to self-assemble into membrane films at water interfaces, held in place by hydrophilic ‘head’ groups. Unlike most molecules, phosphatidylcholines are zwitterions—they contain separate but oppositely charged components; in this case, a head-group with a negatively charged phosphate and a positively charged amine. Researchers suspected that water behaves in unexpected ways at zwitterionic lipid interfaces, and now they have the quantitative details.
Tahara and colleagues used their HD-VSFG technique to make only surface water molecules vibrate at a phosphatidylcholine interface. Instead of the broad oxygen–hydrogen (OH) bond-stretching vibrations normally seen in water, they spotted a distinct, double-peaked feature with both positive and negative components. Tahara notes this is clear evidence of two different surface water structures: an ‘H-down’ orientation, with water’s hydrogen atoms pointing away from the lipid interface, and the opposite ‘H-up’ arrangement.
To pinpoint how these two structures arranged at zwitterionic interfaces, the researchers mixed together pure anionic and cationic lipid surfactants in varying amounts. Then using HD-VSFG, they revealed that H-up structures attached to negative phosphates through hydrogen bonding, while H-down structures bonded to positively charged amines—a new concept for phosphatidylcholine interfaces (Fig. 1).
The researchers also saw a third distinct OH vibrational signal: a water structure with H-up orientation in the hydrophobic lipid tail. “We think that insights from this study can lead to better understanding and predictions of protein structure as well as electron transfer processes within cellular membranes,” Tahara concludes.
References
- 1. Nihonyanagi, S., Yamaguchi, S. & Tahara, T. Direct evidence for orientational flip-flop of water molecules at charged interfaces: A heterodyne-detected vibrational sum frequency generation study. Journal of Chemical Physics 130, 204704 (2009). doi: 10.1063/1.3135147
- 2. Mondal, J.A., Nihonyanagi, S., Yamaguchi, S. & Tahara, T. Three distinct water structures at a zwitterionic lipid/water interface revealed by heterodyne-detected vibrational sum frequency generation. Journal of the American Chemical Society 134, 7842–7850 (2012). doi: 10.1021/ja300658h
