Mar. 14, 2014 Perspectives Physics / Astronomy
The magic of nuclear physics
Powerful machines known as accelerators allow physicists to study how the elements form and also to create new elements and atomic isotopes.
Hideto En’yo
Director
RIKEN Nishina Center for Accelerator-Based Science
Except for hydrogen, all other chemical elements originated from violent nuclear processes in stars. Powerful machines known as accelerators allow physicists to study how these elements form and also to create new elements and atomic isotopes. As such, employing accelerators to study atoms not only improves our understanding of the Universe, but also produces synthetic isotopes useful in applications that include medical diagnostics.
 © 2014 RIKEN
© 2014 RIKEN
Shortly after the Big Bang, the Universe consisted only of hydrogen—the smallest of the chemical elements—and its electrically uncharged partner, the neutron. The diversity of chemical elements that have come into existence since then resulted from nuclear processes taking place within stars. The element helium soon followed hydrogen, forming from the fusion of two hydrogen atoms, and then came carbon, which forms upon the fusion of three helium atoms. Heavier elements, including iron—the most energetically stable element—are created at the end of the life of a star. And elements heavier than iron appear only as a product of catastrophic stellar processes such as supernovae.
Nuclear physicists are using powerful accelerators to shed light on the mechanisms underpinning the Universe’s violent beginnings. Such accelerators can create new atoms with exotic, unstable nuclei that had vital roles in the creation of heavier elements during the explosion of stars.
The dawn of synthetic nuclear physics
Nuclear physics came of age as a research field in the 1920s, when nuclear particles, such as the proton, were discovered. Initial interest focused on understanding the properties of these particles and their involvement in the fundamental nuclear processes that were also discovered at the time: fission and fusion.
Yoshio Nishina was a pioneer of nuclear research in Japan. Following his education at the University of Tokyo and research stints in Europe in the 1920s, Nishina became a chief scientist at RIKEN in 1931. His mission was to establish a nuclear physics laboratory.
One of Nishina’s key research areas—and still the subject of ongoing research—was the study of different nuclear isotopes. Atomic cores consist of two types of particles: the proton, the number of which determines the identity of a chemical element; and the neutron, which is electrically neutral and determines an element’s isotopes. Hydrogen, for example, has one proton and three different isotopes; but heavier chemical elements, such as uranium, can have many different isotopes.
 © 2014 RIKEN
© 2014 RIKEN
The stability of an isotope strongly depends on its mix of neutrons and protons, and this mix also influences how one element transforms into another during a nuclear reaction. A radioactive element, such as uranium, can follow many different reaction pathways to become a more stable isotope. Even the radioactive decay rates of an element can vary greatly between isotopes, ranging from billions of years to a fraction of a second.
An ideal way to study isotope properties is by synthesizing isotopes in the laboratory with the help of accelerators. These machines accelerate and smash atoms together. At sufficiently high energy, the collision of atoms creates new isotopes, or even new elements. Using the first Japanese accelerator—a cyclotron—Nishina discovered a new isotope of uranium, uranium-237, which differed from the form typically found in nature, uranium-238 (containing 92 protons and 146 neutrons).
Nishina built two cyclotrons at RIKEN but both were destroyed after the Second World War. New cyclotrons followed at RIKEN and the latest, the ninth cyclotron, is the world’s most powerful superconducting ring cyclotron (SRC). The SRC can accelerate heavy atomic nuclei to speeds of up to 70 per cent of the velocity of light and has a beam intensity that is 100 times greater than any other accelerator in the world.
The SRC is part of the nuclear research facility at RIKEN that now bears Nishina’s name—the RIKEN Nishina Center for Accelerator-Based Science (RNC). Inaugurated in 2006, the center hosts some 200 full-time scientists and collaborates with many international institutions.
Demystifying ‘magic numbers’
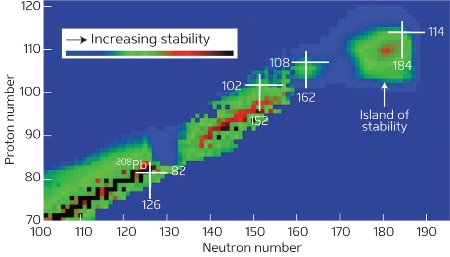 Figure 1: The island of stability The white crosses indicate isotopes with ‘magic’ proton or neutron numbers, for example lead-208 (208Pb), which consists of two magic numbers—82 protons and 126 neutrons—making it especially stable. While very heavy atoms with proton numbers in the hundreds are highly unstable, nuclear physicists predict that even some of the heaviest atoms have magic numbers and form an ‘island of stability’. The as-yet-undiscovered ‘doubly magic’ nucleus with 114 protons and 184 neutrons is presumed to be located on this island. © Yuri Oganessian
Figure 1: The island of stability The white crosses indicate isotopes with ‘magic’ proton or neutron numbers, for example lead-208 (208Pb), which consists of two magic numbers—82 protons and 126 neutrons—making it especially stable. While very heavy atoms with proton numbers in the hundreds are highly unstable, nuclear physicists predict that even some of the heaviest atoms have magic numbers and form an ‘island of stability’. The as-yet-undiscovered ‘doubly magic’ nucleus with 114 protons and 184 neutrons is presumed to be located on this island. © Yuri Oganessian
Experiments using the RNC’s latest generation of cyclotrons are pushing frontiers in nuclear research, particularly in the search for new isotopes with so-called ‘magic numbers’. These isotopes have a set number of protons or neutrons in their nuclei and are surprisingly stable in comparison to other isotopes. Calcium-54, for example, consists of 20 protons and 34 neutrons. This isotope is very unusual: typically, 34 neutrons do not constitute a ‘magic’ isotope; and, its magic properties were theoretically proposed shortly before their experimental discovery by researchers at RIKEN1.
Explaining how elements as heavy as uranium could have resulted from stellar explosions hinges on understanding magic numbers and the stability of their associated elements.
The intense beams of atomic isotopes generated by RIKEN’s cyclotrons are useful in the search for very heavy elements. As heavy isotopes are accelerated and collide with each other, they re-assemble into different atoms, allowing the discovery of new chemical elements. Such research led to the discovery in 2012 of element 113 at the RNC2, after more than nine years of thorough searching.
The synthesis of new chemical elements could lead to a new understanding of nuclear physics. Very heavy atoms are highly unstable and have a very short life, making it difficult to prove their existence following high-energy collisions. However, nuclear physicists predict that even some of the heaviest atoms have magic numbers and could survive for longer after a collision. They are predicted to form a so-called ‘island of stability’ within the short-lived heavy elements, which is typically the domain of elements with approximately 120 protons (Fig. 1).
Since existing accelerators lack the power necessary to reveal new elements within this island of stability, RIKEN plans to replace its synchrotrons 5 and 6 with a new superconducting accelerator. This powerful accelerator would produce beam intensities some 100 times greater than presently possible, securing RIKEN’s leadership in the study of heavy isotopes.Nuclear research for better living
Atomic accelerators also have application beyond fundamental physics. In medicine, for example, certain radioactive isotopes are used as markers in diagnostic experiments because their distribution within the body can be precisely measured. Many of these isotopes are a by-product of uranium fission in nuclear reactors. As research reactors are being decommissioned, alternative methods to produce radioactive isotopes, including accelerators, have become increasingly important.
The use of high-energy ion beams further extends to agriculture. Naturally occurring radioactivity is one of the causes of mutations in plants. Radioactive rays can destroy DNA or cause small changes to the genome. Over many generations, such changes can accumulate and influence an organism’s evolution.
With ion beams, this process can be sped up by introducing mutations at a faster rate. Careful selection of useful mutations in each generation can produce better plants for cultivation. Developing rice that is tolerant to salty water, or even mixtures containing up to 50 per cent sea water, is one example. Controlled mutations could become increasingly important in ensuring a sufficient food supply for an ever-increasing global population.
After more than 80 years of nuclear physics research at RIKEN, much work is still required to better understand the properties of magic isotopes, the formation of heavy elements, the interplay of protons and neutrons in the nucleus and the internal structure of protons and neutrons. Attaining this understanding will require accelerators with even higher energies and intensities than are presently available. In that quest, the RNC will continue to play an important role. The new generation of accelerators being planned will help us to understand how the elementary particles in an atom interact with each other to form the world around us.
References
- 1. Steppenbeck, D., Takeuchi, S., Aoi, N., Doornenbal, P., Matsushita, M. et al. Evidence for a new nuclear ‘magic number’ from the level structure of54Ca. Nature 502, 207–210 (2013). doi: 10.1038/nature12522
- 2. Morita, K., Morimoto, K., Kaji, D., Haba, H., Ozeki, K.et al. New result in the production and decay of an isotope,278113, of the 113th element. Journal of the Physical Society of Japan 81, 103201 (2012). doi: 10.1143/JPSJ.81.103201
