Oct. 31, 2023 Research Highlight Biology
Atomic picture of dengue replication could transform antiviral approaches
Three-dimensional structures of the replication machinery of the dengue virus could lead to new, targeted antiviral drugs
A detailed atomic exploration into how the dengue virus replicates its genome could catalyze the development of high-precision targeted antiviral therapeutics1.
Dengue is one of the most common infections that spread from mosquitoes to people, causing fevers and aches that can sometimes lead to life-threatening complications.
Some drug candidates are being developed that target different proteins involved in the replication of the dengue virus. But a better understanding of how the virus assembles and orchestrates its replication could unlock new avenues for developing more-effective alternatives.
Now, by harnessing the power of cryo-electron microscopy, RIKEN scientists have generated 3D images that reveal the positions of key viral proteins as they adhere to RNA molecules at various stages during the replication cycle of the mosquito-borne virus.
The structures unveiled by this cutting-edge approach help to illuminate a biological process that has long puzzled scientists and eluded drug-discovery efforts.
“We have elucidated part of the mechanism by which the virus propagates in infected cells,” says Shun-ichi Sekine of the RIKEN Center for Biosystems Dynamics Research. “These findings could lead to better antiviral drugs for dengue virus infections.”
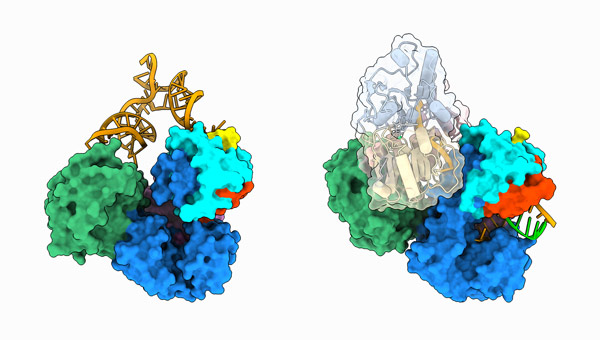
Figure 1: Structures of dengue virus replication complexes, and the initiation of genome duplication (left) and during RNA elongation (right). © 2023 RIKEN Center for Biosystems Dynamics Research
Sekine and his team determined how two critical viral proteins—an RNA-copying enzyme called NS5 and an RNA-unwinding enzyme called NS3—interface with a small strand of RNA designed to mimic the dengue genome.
The researchers focused on two stages of viral replication: at the beginning of RNA synthesis and during RNA elongation. Their structural analyses revealed not only the intricate mechanics behind how the dengue virus copies its genome, but also the molecular foundations that drive NS5’s multifaceted functions during infection. A versatile protein, NS5 can suppress the antiviral capabilities of immune signaling molecules in the human host by binding to them.
One RNA–protein interaction in particular—between NS5 and a segment of the dengue genome known as stem loop A—could prove particularly amenable to drugging with small-molecule therapeutics. The interaction, which is essential to get the replication process started, creates a physical groove termed a ‘thumb pocket’—exactly the type of feature that drug developers look for when designing medications.
A drug that binds at that site could dislodge NS5 and halt dengue replication in its tracks. And because this same structural element is found in the genomic RNA of related viruses, including the ones responsible for Zika infections and Japanese encephalitis, the findings could help to inspire new therapeutics for flaviviruses of all kinds.
Related content
- Gene-reading enzyme razes and rebuilds DNA-winding structures in its path
- A viral inhibitor of cellular stress response shows therapeutic potential
- Elongation factors smooth transcription in the nucleosome
Rate this article
Reference
- 1. Osawa, T., Aoki, M., Ehara, H. & Sekine, S.-I. Structures of dengue virus RNA replicase complexes. Molecular Cell 83, 2781–2791 (2023). doi: 10.1016/j.molcel.2023.06.023
