Jan. 19, 2024 Research Highlight Chemistry
Making useful compounds directly from nitrogen in the air
Molecular structures used in drugs can be made directly from nitrogen in the air, eliminating the need to make ammonia first
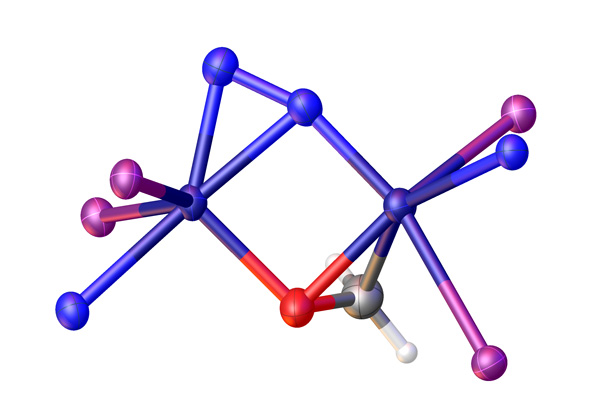
Figure 1: This simplified chemical structure shows how dinitrogen (two blue spheres at top center) is bound to titanium ions (two navy spheres on left and right corners of square) in a complex that includes other ligands based on phosphorus (purple), nitrogen (blue), carbon (silver) and oxygen (red). Adapted, with permission, from Ref. 1. Copyright 2024 American Chemical Society.
RIKEN chemists have discovered a titanium-based compound that can activate nitrogen gas in the atmosphere so that it reacts with certain carbon-based organic molecules, forming potentially useful products1.
All the nitrogen atoms in pharmaceuticals, agrochemicals and synthetic materials originally come from the air around us. The atmosphere is a vast reservoir of nitrogen, with the gas in the form of ‘dinitrogen’ (N2) making up 78% of the atmosphere.
With nitrogen all around us, it might seem easy to pluck it from the air to make industrial compounds. The trouble is that the two nitrogen atoms in dinitrogen are bound by an extremely strong triple bond that requires a lot of energy to break.
Consequently, the main way that nitrogen is sourced from the air is by the energy-intensive Haber–Bosch process, which combines nitrogen and hydrogen gases at high temperature and pressure to form ammonia. Ammonia is then used to make a plethora of organic molecules that are ingredients in medicines, materials, and many other things.
But chemists have long been seeking alternative methods that could bypass ammonia by using nitrogen directly from dinitrogen. This approach would offer shorter synthetic routes and reduce our reliance on the Haber–Bosch process. As yet, very few reactions can do this.
However, a team led by Zhaomin Hou of the RIKEN Center for Sustainable Resource Science has now developed a novel reaction process with mild conditions that can directly incorporate nitrogen from the air into compounds.
Previously, Hou’s team had made a compound that could bind dinitrogen. It contained two titanium atoms, surrounded by various chemical groups called ligands.
Now, they have added carbon monoxide to this compound, and found that it creates a new ligand called oxymethylene. This helps the titanium atoms to hold the dinitrogen unit together and enables it to participate in chemical reactions.
The team mixed the new compound with a range of organic molecules that had a carbon–carbon double bond close to a carbon–oxygen double bond. The new titanium compound added dinitrogen to these molecules to create products that included ring-shaped molecules called pyrazolidinones and pyrazolines. These rings contain a pair of adjacent nitrogen atoms, and are a key motif in some pharmaceuticals and agrochemicals.
Pyrazolidinones and pyrazolines are usually synthesized using a nitrogen-containing reagent called hydrazine, which is itself made from ammonia. While making these products from dinitrogen is a more direct route, the method is unlikely to supersede the well-established and cost-effective Haber–Bosch process, Hou says.
“The direct use of dinitrogen for synthesizing nitrogen-containing organic compounds will remain a long-standing research subject,” he says, adding that his team is now exploring alternative ways to use dinitrogen as a reagent.

A team led by Zhaomin Hou (fourth from right in second row) has developed a titanium-based compound that can activate dinitrogen from the air. © 2024 RIKEN
Related content
- A step toward unlocking the nitrogen reservoir in the sky
- Breaking benzene
- An energy-efficiency lead for nitrogen fertilizer production
Rate this article
Reference
- 1. Zhuo, Q., Yang, J., Zhou, X., Shima, T., Luo, Y. & Hou, Z. Aza-Michael addition of dinitrogen to α,β-unsaturated carbonyl compounds in a dititanium framework. Journal of the American Chemical Society 145, 22803−22813 (2023). doi: 10.1021/jacs.3c08715
