Aug. 16, 2010 Press Release Biology Chemistry Medicine / Disease
Small-molecule inhibitor uncovers protein role in melanoma cell migration
A nuclear protein of previously unknown function has been shown to regulate the migration of tumor cells in the spread of melanoma, the most deadly form of skin cancer. Researchers at the RIKEN Advanced Science Institute made the discovery by means of a small-molecule inhibitor they identified using a powerful new chemical array screening technique.
Characterizing the functions of proteins in the cell, whose role in mediating complex metabolic and signaling networks is central to cellular biochemistry, is essential for developing new medicines and treatments. Small-molecule inhibitors have proven an effective tool for doing this, binding to target proteins and disrupting their normal function in order to reveal their network of cellular interactions.
Human pirin is a nuclear protein known to play a role in a variety of biological processes, yet one with a function which remains unclear. To identify inhibitors for this protein, the team used a technique they developed called chemical array screening, in which many small-compound molecules are immobilized onto glass slides and incubated with the target protein (Figure 1). From more than 20,000 molecules screened, the team identified one they named triphenyl compound A (TPh A) that binds to pirin with high affinity.
Using X-ray crystallography, the team determined how TPh A binds to pirin at a resolution of 2.35 Å (Figure 2). They went on to show that TPh A inhibits interaction between pirin and its binding partner, Bcl3, and that it also inhibits the migration of melanoma cells by reducing expression of the tumor mobility protein SNAI2.
Reported in Nature Chemical Biology, the findings establish for the first time the role of pirin in melanoma cell migration and elucidate its structure through its binding with TPh A. They also demonstrate the power of chemical array screening, whose further application promises to greatly expand our understanding of proteins and their interactions in the cell.
Reference
- Isao Miyazaki, Siro Simizu, Hideo Okumura, Satoshi Takagi and Hiroyuki Osada. A small-molecule inhibitor shows that pirin regulates migration of melanoma cells. Nature Chemical Biology (2010).
Contact
Hiroyuki Osada
Chemical Biology Core Facility, Advanced Computational Sciences Department
RIKEN Advanced Science Institute
Tel: +81-(0)48-467-9542 / Fax: +81-(0)48-467-4669
Jens Wilkinson
RIKEN Global Relations and Research Coordination Office
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: pr@riken.jp
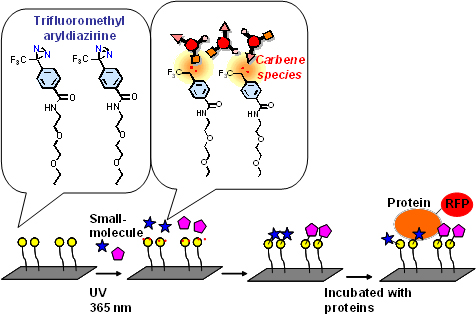
Figure 1: Overview of a chemical array screening
In this screen, small molecules are covalently immobilized onto glass slides using a photoaffinity linker containing trifluoromethyl aryldiazirine. Under UV radiation, aryldiazirine moiety produces carbine species, known for their high reactivity. The carbine species are so reactive that they react with every type of small molecule. The immobilized small molecules retain their ability to interact with their binding proteins. Proteins tested are labeled with RFP to detect fluorescence signals.
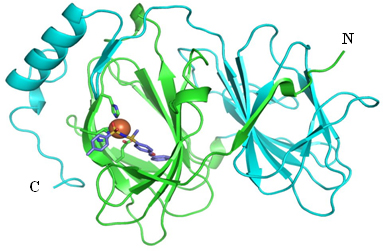
Figure 2: Crystal structure of the complex formed by pirin and TPh A
The N- and C-terminal domains are cross-linked, with a single iron ion (brown) in the N-terminal domain. TPh A is shown as a stick model.
