Aug. 4, 2006 Research Highlight Biology
Molecular mimic sets the tone for calcium release
A new model of calcium release in cells provides insight into how intracellular communications are regulated
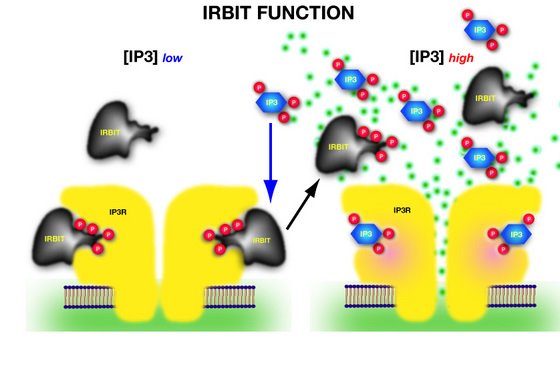 Figure 1: IRBIT regulates activity through direct competition for the binding site on the IP receptors. Once IP3 reaches a threshold concentration, it displaces IRBIT from the binding site (P = phosphate group indicating phosphorylation status; green dots = calcium ions).
Figure 1: IRBIT regulates activity through direct competition for the binding site on the IP receptors. Once IP3 reaches a threshold concentration, it displaces IRBIT from the binding site (P = phosphate group indicating phosphorylation status; green dots = calcium ions).
Cells constitute exquisitely fine-tuned systems whose development, function and death is determined by the well-orchestrated interplay of numerous cell processes. Maintaining these systems requires good communication between different cell components.
Cells respond to external and internal signals by transferring information from the ‘receiving antennae’ on a membrane, the so-called receptors, to appropriate targets using ‘signaling’ molecules.
Calcium is probably the most abundant of these signaling molecules. It can enter the cell or be released from storage compartments within the cell via specialized calcium-specific channels.
Katsuhiko Mikoshiba, a neurobiologist at RIKEN’s Brain Science Institute in Wako, and colleagues from the University of Tokyo and the Japan Science and Technology Agency, now propose, in Molecular Cell 1, a model to explain the regulation of the activity of one family of internal calcium-specific channels, the inositol-trisphosphate receptors (IP3Rs).
Working with mouse, monkey and human cells, the team determined how the activity of the natural trigger of calcium release by the IP3Rs, inositol-trisphopshate (IP3), is modulated by IRBIT, a previously described IP3-binding protein2. Mikoshiba and colleagues show that at the molecular level, the interaction of IRBIT with the IP3Rs is based on a similar structure and phosphorylation status of the components in IRBIT and IP3 that interact with the receptor—in other words, IRBIT mimics and competes with IP3 (Fig. 1).
IRBIT alone however has no function and does not trigger the release of calcium, which led the researchers to postulate that: “The physical competition between IRBIT and IP3 for binding with the IP3Rs primarily determines a threshold concentration above which IP3 has to be present for calcium to be released into the cell. IRBIT thus regulates the oscillation in IP3-dependent calcium concentrations, which in turn modulates gene expression and enzyme activity.”
While the team has previously identified the interaction of IRBIT with the IP3Rs, no exact mechanism of interaction was known and no regulatory model had been proposed2. “Our current work sheds new light on how cells have evolved to regulate the complex task of intracellular communication by using seemingly simple, yet very sophisticated, molecular tricks,” says Mikoshiba.
Final confirmation of the validity of the proposed model will come through crystallographic analysis of the IP3Rs bound to IRBIT. And, further studies are required to determine the physiological importance of the interaction between IRBIT and IP3R.
References
- 1. Ando, H., Mizutani, A., Kiefer, H., Tsuzurugi, D., Michikawa, T., & Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Molecular Cell 22, 795–806 (2006). doi: 10.1016/j.molcel.2006.05.017
- 2. Ando, H., Mizutani, A., Matsu-ura, T., & Mikoshiba, K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. Journal of Biological Chemistry 278, 10602–10612 (2003). doi: 10.1074/jbc.M210119200
