Sep. 1, 2006 Research Highlight Biology
A new recipe for making neurons in a dish
A readily available human material supports neural differentiation of human embryonic stem cells
 Figure 1: Culture dishes in which matrix materials were coated.
Figure 1: Culture dishes in which matrix materials were coated.
Recently, the possibility of using cells differentiated from embryonic stem (ES) cells in the laboratory to ameliorate debilitating human diseases has garnered much media attention. Yoshiki Sasai and colleagues at the RIKEN Center for Developmental Biology, in Kobe, developed a new system to stimulate ES-to-neuron differentiation in a culture dish.
The therapeutic utility of ES cells stems from their ‘pluripotency’, or their ability to become any type of cell. Different tissues within the body are composed of distinct cell types; each specialized to perform the specific set of functions demanded in that tissue. Some diseases affect tissues that can be replaced by organs obtained from other human donors. However, other diseases result in damage not so easily undone. For example, Parkinson’s disease causes progressive irreversible damage to neurons. Amelioration of the resultant devastating symptoms therefore requires a source of healthy replacement neurons.
Producing wholly human neurons
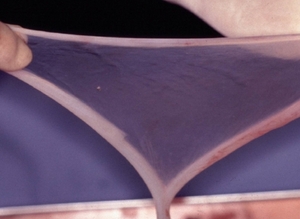 Figure 2: Amniotic membrane detached from the human placenta.
Figure 2: Amniotic membrane detached from the human placenta.
While neurobiologists know that embryonic pluripotent cells hold the potential to become neurons, they do not understand precisely how this occurs inside the body. This limited understanding renders the task of inducing and recapitulating this mysterious process outside of the body, in the artificial environment of a laboratory dish, or in vitro, all the more daunting.
Despite these obstacles, researchers have devised a number of systems capable of supporting human ES cell to neural cell differentiation in vitro. As demonstrated in preliminary studies, neurons generated using these systems exhibit therapeutic activity in a monkey model of Parkinson’s disease.
The most popular system used for in vitro production of neurons involves ES cell culture on supportive mouse cells called stromal cells. Although capable of generating multiple types of neurons, one flaw inherent in this system—the use of non-human material—will likely preclude its clinical application. Human precursor cells differentiated on non-human stromal cells can ‘pick up’ bits of non-human material. The human immune system, in its perpetual patrol for ‘non-self’ material, recognizes these precursor cells as foreign and orchestrates their elimination.
Sasai and colleagues demonstrated that mouse and human ES cells cultured in dishes (Fig. 1) coated with matrix layers of human amniotic membrane (hAM) (Fig. 2)—the internal coating of the human placenta—gave rise to diverse types of neurons. These neurons displayed surface proteins characteristic of those decorating the surface of neurons located in multiple regions of the brain (Fig. 3). To ensure that this resemblance was more than superficial, Sasai and colleagues analyzed the spectrum of genes expressed within neural precursors differentiating in dishes coated with hAM; these neural precursors expressed genes found in bona fide neurons. In addition, neural precursor cells differentiating on the hAM matrix responded to external stimuli in a manner similar to neural precursor cells developing within a live mouse or human embryo.
Thought-provoking findings
 Figure 3: Neural cells generated from human ES cells using the hAM in vitro system. Green is a neural marker and blue is the nucleus. Neural cells make rosette-like structures.
Figure 3: Neural cells generated from human ES cells using the hAM in vitro system. Green is a neural marker and blue is the nucleus. Neural cells make rosette-like structures.
In experiments using mouse stromal cells, a portion of ES cells incubated with hAM developed into what appeared to be retinal pigment epithelial cells and lens cells, which are crucial components of eye tissue. Importantly, Sasai’s team excluded the possibility that the observed neural differentiation was due to contamination with pre-committed neural cells, or to fusion between stromal cells and differentiating ES cells. Their work is reported in the Proceedings of the National Academy of Sciences1.
Sasai and colleagues tested other matrix materials such as gelatin, collagen, and fibronectin, but none of these materials supported ES-to-neuron development as efficiently as hAM. These results highlight the unique, yet still-not-understood capabilities of hAM1.
Of course, the ultimate test is to determine whether the neurons produced in this new system, which from every angle look like bona fide neurons, act like bona fide neurons. The team also needs to assess whether these neurons exhibit therapeutic activity in animal models of Parkinson’s disease.
According to Sasai, the planning of these experiments is already under way. "In collaboration with Kyoto University Hospital, we plan to graft these cells into the basal ganglia of Parkinsonian model monkeys. This is an important and, in a sense, the ultimate step of preclinical studies of stem cell therapy for this disease,” says Sasai. Because the neurons generated on hAM strongly resemble those generated on mouse stromal cells, which did exhibit therapeutic activity in a monkey model of Parkinson’s disease, the researchers have reason to be optimistic.
The ready availability of hAM via informed consent during caesarean section is a plus for the team. Further, its biological safety has been proven after years of use in opthalmological and dermatological surgeries, and its neural differentiative capability was maintained even after six months of frozen storage. “How and why the amniotic membrane has such activity is a mystery, but, practically speaking, it works ideally for stem cell therapy,” says Sasai.
Remaining questions
A remaining challenge for the team is to identify the cell type(s) and factor(s) derived from the hAM matrix layer that are responsible for inducing ES-to-neuron development. This information might help in gaining a more thorough understanding of the stimuli and signals that induce neural differentiation within the body.
Sasai and colleagues failed to identify an individual hAM component that could alone support ES-to-neuron differentiation. But as stem cell research continues to attract media attention globally, the team's findings represent another step forward in the clinical application of human cells differentiated in the laboratory.
References
- 1. Ueno, M., Matsumura, M., Watanabe, K., Nakamura, T., Osakada, F., Takahashi, M., Kawasaki, H., Kinoshita, S. & Sasai, Y. Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proceedings of the National Academy of Sciences USA 103, 9554–9559 (2006). doi: 10.1073/pnas.0600104103
About the Researcher
Yoshiki Sasai

Yoshiki Sasai was born in Hyogo, Japan in 1962 and received his MD from Kyoto University School of Medicine in 1986. From 1986 to 1988, he performed an internship in general and emergency medicine. In 1993, he earned a PhD from the same university for work on neural specific transcriptional regulators. From 1993 to 1996, he was a visiting research fellow at UCLA School of Medicine, and then appointed as an associated professor at Kyoto University School of Medicine. From 1998 to 2003, he served as a professor at Institute for Frontier Medical Sciences, Kyoto University, while becoming a group director at the RIKEN Center for Developmental Biology in 2000. He has been concurrently serving as an affiliated professor at Kyoto University School of Medicine since 2003.
