Dec. 1, 2006 Research Highlight Biology
Insights into brain architecture
Cross talk between neurons helps generate the cerebellum
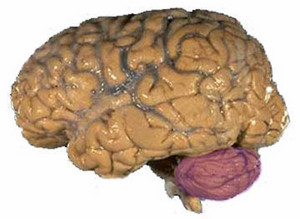 Figure 1: The human brain, with the large cerebrum and smaller cerebellum (purple).
Figure 1: The human brain, with the large cerebrum and smaller cerebellum (purple).
Interactions between cells in the cerebellum—or ‘little brain’—are essential for normal function. A collaboration of Japanese researchers has now found that the proper development of the tree-like morphology of Purkinje cells in the cerebellum requires signals from other cerebellar cells called granule cells.
The cerebellum is a region of the brain (Fig. 1) that coordinates sensory information. Lesions in the cerebellum cause subtle defects in movement and balance, and some evidence suggests conditions such as schizophrenia are caused by cerebellar dysfunction.
Unique to the cerebellum are the specialized Purkinje cells (Fig. 2)—named for the Czech anatomist Jan Purkynĕ. Development of the great numbers of Purkinje cell ‘branches’ or dendrites, which project into the outer layer of the cerebellum where they interact with other neurons such as parallel fibers, is essential for cerebellar function.
Remarkably, although the cerebellum represents only about 10% of total brain size, the number of cerebellar neurons constitutes some 50% of total brain neurons. Upwards of 60 billion granule cells occupy the inner region of the cerebellum.
The collaboration of neuroscientists led by Katsuhiko Mikoshiba and Chihiro Hisatsune of the RIKEN Brain Science Institute, Wako, found that signals produced by granule cells are required for proper growth of the complex Purkinje cells1. The team evaluated mice that lack inositol 1,4,5-triphosophate receptor type 1 (IP3R1), a signaling protein found throughout the brain.
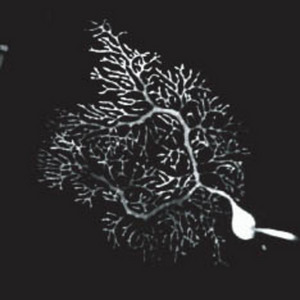 Figure 2: A Purkinje cell. PNAS/National Academy of Sciences/26/10922 (2006)
Figure 2: A Purkinje cell. PNAS/National Academy of Sciences/26/10922 (2006)
By studying isolated Purkinje cells and granule cells from normal mice and mice lacking IP3R1, the team showed that granules cells, not Purkinje cells, need IP3R1 in order to produce brain-derived neurotrophic factor (BDNF), a growth factor that Purkinje cells need for development of their tree-like dendrites.
The team also visualized dendrite branches of Purkinje cells in brains of mice that lack IP3R1. Compared to normal mice, the IP3R1-deficient brains showed reduced connections between Purkinje cells and parallel fibers in the outer layer of the cerebellum, clearly implicating IP3R1 in regulating normal cerebellar development.
“We now want to know the molecular mechanism by which IP3R1 regulates BDNF expression from granule cells,” says Mikoshiba. “And because IP3R1 is found in many regions of the brain, future work will hopefully reveal the physiological roles of IP3R1 in cells forming these other regions as well.”
Understanding the architecture of the brain requires understanding how individual neurons interact with one another. Mikoshiba and colleagues’ work highlights the complex interactions between cerebellar Purkinje cells and granule cells that are required for forming correct connections between them.
References
- 1. Hisatsune, C., Kuroda, Y., Akagi, T., Torashima, T., Hirai, H., Hashikawa, T., Inoue, T. & Mikoshiba, K. Inositol 1,4,5-triphosphate receptor type 1 in granule cells, not in Purkinje cells, regulates the dendritic morphology of Purkinje cells through brain-derived neurotrophic factor production. The Journal of Neuroscience 26, 10916–10924 (2006). doi: 10.1523/JNEUROSCI.3269-06.2006
