Mar. 2, 2007 Research Highlight Biology
How surface cells band together
RIKEN researchers discover a key role for the Tuba protein
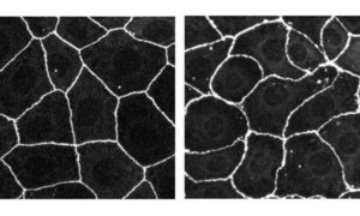 Figure 1: The typical ‘honeycomb’ appearance in an epithelial cell line from the human intestine (left). The distorted appearance when Tuba synthesis is disrupted by RNA interference (right). Copyright 2006 Rockefeller University Press
Figure 1: The typical ‘honeycomb’ appearance in an epithelial cell line from the human intestine (left). The distorted appearance when Tuba synthesis is disrupted by RNA interference (right). Copyright 2006 Rockefeller University Press
Researchers from RIKEN and Kyoto University have unraveled details of how an enzyme known as Tuba regulates the joining together of tissue surface or epithelial cells.
Epithelial cells characteristically form a honeycomb-like structure (Fig. 1) such that membrane contact areas between neighboring cells are held to a minimum. This suggests that the cells pack together under some form of tension. The honeycomb appearance is thought to be important in the functioning of the surfaces—minimizing light scattering in the lenses of the eye, for instance. Formation and remodeling of such surfaces is a significant facet of development.
The cells themselves tend to be columnar in shape. In the region closest to the surface—the apical region—the membranes of neighboring cells are pushed together and adhere to one another in a complex of two kinds of junction. Nearest the surface a tight junction forms, where the membranes are so closely joined they form an impermeable barrier to fluid, preventing the passage of molecules and ions between cells.
Immediately beneath the tight junction is a looser junction, where cell membranes are anchored to each other by complexes of three families of proteins—cadherins, catenins and flexible actin filaments which extend into the bodies of the adjoining cells. Signalling proteins, such as Tuba, can help to organize such complexes by bringing together the interacting proteins.
The research team at RIKEN’s Center for Developmental Biology in Kobe reports in The Journal of Cell Biology 1 that staining studies show Tuba to be concentrated in the tight junction area. When Tuba synthesis is disrupted by RNA interference, the team found, the honeycomb packing of human intestinal epithelial cells becomes flabby, and takes on a curved, distorted appearance (Fig. 1).
Tuba is a guanine nucleotide exchange factor (GEF) known to activate Cdc42, a compound which regulates the assembly of actin filaments and cadherins. The researchers discovered that Tuba inactivation leads to disruption of the network of actin and cadherin fibers involved in holding neighboring membranes together under tension. They suggest that Tuba stimulates Cdc42 and other compounds to enhance assembly and delivery of actin and cadherins to the junction area.
Several studies have implicated other GEFs in the cell-cell adhesion process. “How the different GEFs share the cell assembly regulation roles is an intriguing topic and one we are looking to tackle in the future,” says the paper’s lead author, Tetsuhisa Otani.
References
- 1. Otani, T., Ichii, T., Aono, S. & Takeichi, M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. The Journal of Cell Biology 175, 135–246 (2006). doi: 10.1083/jcb.200605012
