Jun. 1, 2007 Research Highlight Biology
Asymmetric protein distribution mediates cell division
Japanese researchers begin to unravel a key signaling pathway in asymmetric cell division
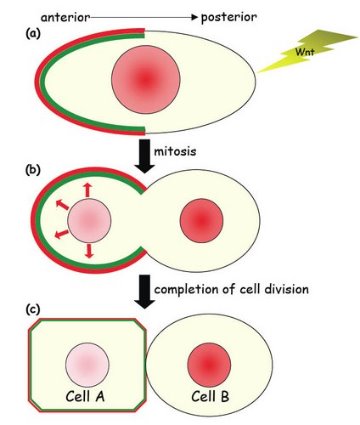 Figure 1: A schematic representation of asymmetric cell division in C. elegans.
Figure 1: A schematic representation of asymmetric cell division in C. elegans.
In many species, the protein ß-catenin plays two roles, in cadherin-mediated cell adhesion and in the Wnt signaling pathway that plays a crucial role in embryogenesis and morphogenesis. The nematode Caenorhabditis elegans has four ß-catenin proteins, one involved in cell adhesion and the other three associated with signaling.
In C. elegans, an atypical Wnt signaling pathway regulates asymmetric cell division, producing two daughter cells with different cell fates. In worms with mutations in this pathway, many cells divide symmetrically and fail to acquire proper cell fate. Mutations of the mammalian Wnt signaling pathway have been associated with tumor formation.
Kota Mizumoto and Hitoshi Sawa, from the RIKEN Center for Developmental Biology, Kobe, are unraveling the Wnt pathway, focusing on the C. elegans ß-catenin protein WRM-1, which localizes to the anterior cortex and the posterior nucleus during cell division, suggesting a role in asymmetrical cell division1.
Initially, the researchers expressed WRM-1 as a fusion protein in a hypodermal cell of the nematode tail, engineered to localize uniformly throughout the cortex, rather than asymmetrically. Symmetric cell division then resulted in two hypodermal daughter cells—rather than a hypodermal and neural cell. This was also observed in mutant worms lacking the wrm-1 gene, suggesting the uniform localization of the protein caused the defect in asymmetric cell division.
Next, they examined components of the ‘destruction complex’, which plays an important role in regulating ß-catenin activity by binding to ß-catenin when the signaling protein Wnt is not present. Inhibition or removal of one factor in the destruction complex, the APC homolog APR-1, caused both daughter cells to become neural cells, not hypodermal cells. The researchers also observed that APR-1, and another component of the destruction complex, PRY-1, localize to the anterior cortex, possibly together with WRM-1. Mizumoto and Sawa hypothesize that APR-1 is recruited by WRM-1 to the anterior cortex before and during cell division, while cortical APR-1 stimulates the export of nuclear WRM-1 to the anterior cortex at telophase, the final stage of cell division (Fig. 1).
“So far, it is not clear whether Wnt signaling regulates asymmetric cell division in mammalian cells,” says Mizumoto. “However, the Wnt pathway is known to be required for stem cell maintenance. Because stem cells are thought to undergo asymmetric cell division, it is interesting to hypothesize that Wnt signaling maintains stem cell through regulating its asymmetric cell division as in C. elegans.”
References
- 1. Mizumoto, K. & Sawa, H. Cortical ß-catenin and APC regulate asymmetric nuclear ß-catenin localization during asymmetric cell division in C. elegans. Developmental Cell 12, 287–299 (2007). doi: 10.1016/j.devcel.2007.01.004
