Jun. 1, 2007 Research Highlight Chemistry
Molecular double act in crystals
Conducting and magnetic electrons co-exist in crystals containing two differently arranged layers of the same metal complex
The properties of a material not only depend on what it is made from, but also on how its atomic (or molecular) building-blocks are arranged. For example, diamond and graphite are two very different substances, but each is made only from carbon atoms. The different organization of the atoms in each material have profound implications for their properties—diamond is an electrical insulator and one of the hardest substances known, whereas graphite is a very soft material that conducts electricity.
Although he uses more exotic building-blocks than carbon atoms, Reizo Kato from RIKEN’s Discovery Research Institute in Wako is studying similar effects in molecular crystals. “The electronic properties of these materials can be strongly influenced by their structure,” says Kato, “a molecular assembly can be conducting or insulating, depending on how the individual molecules are organized.”
In their most recent work in this area1, Kato and colleagues investigate how the arrangement of a nickel-containing metal complex—widely used in conducting or magnetic materials—can be controlled in a crystal. These molecules have sulfur atoms at both ends, which are known to form intermolecular interactions—known as ‘halogen bonds’—with iodine atoms.
In what Kato describes as a breakthrough, a graduate student in his laboratory, Yosuke Kosaka, made a salt from the negatively charged nickel complex and a positively charged molecule containing two iodine atoms. The structure of this compound was investigated using x-ray crystallography, which revealed that the iodine–sulfur interactions direct the nickel complexes to form two kinds layers—with very different molecular arrangements—that alternate (Fig. 1) throughout the crystal.
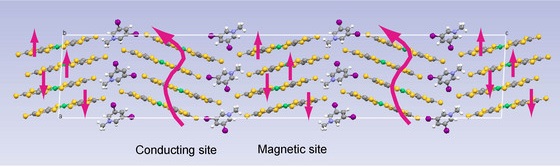 Figure 1: The structure of a ‘Jekyll and Hyde’ molecular crystal in which alternating layers of the same nickel complex (shown in yellow, green and grey) have different magnetic and electrical properties. The iodine atoms of the cationic ‘glue’ that holds the layers together are shown in purple.
Figure 1: The structure of a ‘Jekyll and Hyde’ molecular crystal in which alternating layers of the same nickel complex (shown in yellow, green and grey) have different magnetic and electrical properties. The iodine atoms of the cationic ‘glue’ that holds the layers together are shown in purple.
In one layer, the nickel complexes are stacked on top of one another and group together in strongly associated pairs called dimers. This layer acts as an electrical insulator and electron spins on neighboring dimers show paramagnetic behavior with a tendency to align in opposite directions—described as antiferromagnetic interaction.
In the other layer, however, the nickel complexes form a non-columnar structure in which some molecules overlap with more than one neighbor and instead span across two. This layer exhibits two-dimensional metallic conduction down to a temperature of 4.2 K, resulting in an overall crystal that contains alternating insulating and conducting layers.
These two layers are composed of the same molecule, yet their physical properties are remarkably different because of the dissimilar molecular arrangements. “This is a molecular version of Dr Jekyll and Mr Hyde,” says Kato, “and is the first example of such behavior in molecular crystals.”
References
- 1. Kosaka, Y., Yamamoto, H. M., Nakao, A., Tamura, M. & Kato, R. Coexistence of conducting and magnetic electrons based on molecular π-electrons in the supramolecular conductor (Me-3,5-DIP)[Ni(dmit)2]2. Journal of the American Chemical Society 129, 3054–3055 (2007). doi: 10.1021/ja0687825
