Aug. 3, 2007 Research Highlight Biology
Stimulating retinal repair
Repairing damaged retinas is now a possibility
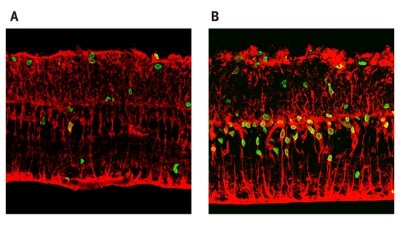 Figure 1: Treatment of damaged retinas with Wnt3a significantly increases the amount of retinal regeneration as shown by the number of BrdU-positive proliferative cells (green). (a) The amount of regeneration that occurs naturally. (b) Retinal regeneration after Wnt3a treatment. © J. Neuorsci./ Society for Neuroscience/ 27(15)/ 4212 (2007)
Figure 1: Treatment of damaged retinas with Wnt3a significantly increases the amount of retinal regeneration as shown by the number of BrdU-positive proliferative cells (green). (a) The amount of regeneration that occurs naturally. (b) Retinal regeneration after Wnt3a treatment. © J. Neuorsci./ Society for Neuroscience/ 27(15)/ 4212 (2007)
Japanese researchers from RIKEN and Kyoto University have demonstrated retinal regeneration in a mammalian model of retinal degeneration after stimulation of the Wnt signaling pathway. In addition to its better known roles in embryogenesis and development, this pathway also functions as a regulator of some adult stem cell populations.
The team’s work may lead to new therapies for retinal diseases including the degenerative disease called retinitis pigmentosa, in which the rod and cone photoreceptor cells of the retina die off, leading to vision impairment and ultimately blindness.
Wnt stimulates regeneration in vitro
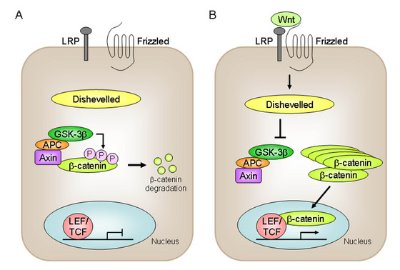 Figure 2: The Wnt signaling pathway activates gene transcription via β-catenin.
Figure 2: The Wnt signaling pathway activates gene transcription via β-catenin.
Previous research by the team, led by Masayo Takahashi at the RIKEN Center for Developmental Biology, Kobe, demonstrated that retinal support cells called Müller glia could de-differentiate, or revert to a cell type from earlier in its developmental pathway, to assume a neuronal fate, but the level of regeneration via this mechanism was very low, occurring in just a few cells.
Now, in an in vitro model of retinal damage, the team has found that retinal cell regeneration can be increased by as much as twenty-fold in the presence of the protein Wnt3a. Their findings are published in the Journal of Neuroscience 1.
The researchers initially performed experiments in cultured retinas isolated from rats, which can be used as a model for retinal damage. In these so-called explant cultures, the retina is cultured as a unit with no dissociation of retinal cells. After four days in culture, a small proportion of the Müller glial cells re-entered the cell cycle. Immunostaining of the cells further demonstrated that some of these cells were expressing proteins that were characteristic of neural stem cells, suggesting that the Müller glial cells were de-differentiating to neural progenitor cells. When they administered the protein Wnt3a, they found a significant increase in proliferation of these neuronal progenitors from the de-differentiated cells (Fig. 1).
“Newly generated cells constituted almost a layer of cells in the outer nuclear layer,” says Takahashi. “We only observed several cells per field without Wnt treatment. Furthermore, the retinal neurons were regenerated all over the retina.”
This process appears to involve the canonical Wnt signaling pathway, in which Wnt activation protects the β-catenin protein from degradation mediated by a glycogen synthase kinase complex, allowing it to accumulate in the nucleus where it regulates gene transcription (Fig. 2). A number of components of the pathway were shown to be present in the retina, including Wnt receptor and co-receptor proteins, glycogen synthase kinase and β-catenin. Treatment of the retinal explant cultures with Wnt3a caused accumulation of β-catenin in the nucleus and a subsequent increase in the expression of the cell cycle-regulating protein cyclin D1, a known target of the Wnt signaling pathway, compared to the levels of cyclin D1 in untreated cultures.
Takahashi and her colleagues also demonstrated that the Wnt signaling pathway was involved in the retinal regeneration process in vivo. Furthermore, the regeneration process could be stimulated with small molecule inhibitors of glycogen synthase kinase-3β, which normally blocks activation of the pathway, and conversely, is blocked by the application of Wnt3a inhibitor.
Next, the researchers showed that the regenerated cells migrated to the outer nuclear layer of the retina, where, in the presence of retinoic acid (a form of vitamin A), they observed differentiation into rod photoreceptor cells as evidenced by the appearance of cells expressing rhodopsin (Fig. 3).
Wnt-induced regeneration in a genetic model of degenerative disease
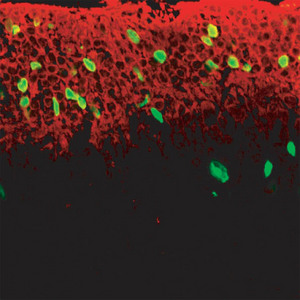 Figure 3: Photoreceptor cells expressing rhodopsin (green) can be seen after treatment of the retinal explant cultures with both Wnt3a and retinoic acid.
Figure 3: Photoreceptor cells expressing rhodopsin (green) can be seen after treatment of the retinal explant cultures with both Wnt3a and retinoic acid.
The team then isolated retinas from a strain of mice with a genetic defect that resembles the human disease retinitis pigmentosa. These mice exhibit a significant loss of photoreceptor cells within one month of birth. Treatment of these retinas with Wnt3a also resulted in the regeneration of retinal cells. This suggests that the Wnt/β-catenin signaling pathway contributes to central nervous system regeneration.
As the researchers had observed in the earlier experiments using retinal explant cultures from rats, a significant increase in cell proliferation was observed after Wnt3a treatment, and the de-differentiated neural progenitors migrated to the outer nuclear layer. But in retinas from mice already exhibiting advanced retinal degeneration, Wnt3a treatment had little or no effect, suggesting that the degree of degeneration prior to treatment affected the ability of the Wnt3a signaling pathway to stimulate repair processes.
Differentiation of the neural progenitor cells into rhodopsin-positive photoreceptor cells was observed following treatment of the Wnt-treated cultures with retinoic acid. A similar result was observed after treatment of the cultures with valproic acid. This compound is an inhibitor of the enzyme histone deacetylase, which regulates expression of another protein involved in photoreceptor development, the transcription factor NeuroD.
Therapeutic possibility
Takahashi believes Wnt signaling may be a part of the natural restoration mechanism in the retina. However, more understanding of the pathway’s role is required before the findings can be turned into therapeutic applications. Proof is also needed that the newly generated photoreceptors are incorporated into the retina, thus restoring its function.
The team’s findings have therapeutic potential for reversing retinal degeneration and repairing damage. If the same regenerative effect can be stimulated in human retinas, small molecule drugs could be used to stimulate the Wnt signaling pathway in situ. Preliminary observations have shown regeneration potential in the primate retina.
“This is the first report to show the possibility of drug induced neural regeneration after damage in the central nervous system,” says Takahashi. “If these newly generated neurons can form synapses and restore function, it will be applied to various diseases or conditions in the central nervous system that do not have therapies at all now.”
References
- 1. Osakada, F., Ooto, S., Akagi, T., Mandai, M., Akaike, A. & Takahashi, M. Wnt signaling promotes regeneration in the retina of adult mammals. Journal of Neuroscience 27, 4210–4219 (2007). doi: 10.1523/JNEUROSCI.4193-06.2007
About the Researcher
Masayo Takahashi

Masayo Takahashi received her MD from Kyoto University in 1986, and her PhD from the same institution in 1992. After serving as the assistant professor in the Department of Ophthalmology, Kyoto University Hospital, Japan, she moved to the Salk Institute, US, in 1996 where her research revealed that stem cells can be used as a tool for retinal therapy. She came back to Kyoto University Hospital in 1998, and since 2001 she has been an associate professor at the Translational Research Center, Kyoto University Hospital. She joined the RIKEN Center for Developmental Biology as a team leader of the retinal regeneration research team in 2006. Her clinical speciality is retinal diseases, especially macular diseases and retinal hereditary diseases. Her aim is to gain a deep understanding of these diseases and to develop retinal regeneration therapy.
