Feb. 1, 2008 Research Highlight Physics / Astronomy
Sticky spins
Researchers show how spins freeze in a molecular magnet
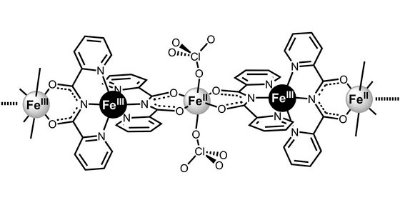 Figure 1: The chemical structure of the molecular magnet catena-[FeII(ClO4)2{FeIII(bpca)2}]ClO4 FeII, high-spin ion; FeIII, low-spin ion; O, oxygen; N, nitrogen; Cl, chlorine © JACS/ American Chemical Society/ 129/ 12360 (2007)
Figure 1: The chemical structure of the molecular magnet catena-[FeII(ClO4)2{FeIII(bpca)2}]ClO4 FeII, high-spin ion; FeIII, low-spin ion; O, oxygen; N, nitrogen; Cl, chlorine © JACS/ American Chemical Society/ 129/ 12360 (2007)
Most of us are familiar with magnets like iron that are strong enough to pull up a paperclip or scramble the colors on a computer monitor. Molecular magnets, on the other hand, are quite different. Compared to the densely packed and strongly interacting spins in permanent magnets, the spins in molecular magnets—usually contributed by iron or manganese ions—are well separated by organic molecules and a different mechanism is responsible for the magnetic state they form at low temperatures.
Molecular magnets are attractive for studying fundamental magnetic properties, particularly because with chemistry, it is possible to change the length or structure of the organic molecules and therefore, the interactions between the spins on the metal ions. Writing in the Journal of the American Chemical Society1, scientists Isao Watanabe at the RIKEN Nishina Center at Wako and colleagues at Tohoku University and The University of Tokyo present an interesting example of this chemical tuning of magnetic properties in a magnetic molecule called catena-[FeII(ClO4)2{FeIII(bpca)2}]ClO4.
This magnetic molecule is a one-dimensional chain consisting of many of the molecular units shown in Figure 1, in which consecutive organic planes twist with respect to one another. The spins on the iron (FeII) ions minimize their energy when they are directed within the molecular planes—an effect known as ‘easy-plane anisotropy’. However, the twisting of the organic planes along the chain actually causes the spins on the FeII ions to collectively point along the chain direction, forming a ‘single-chain’ magnet. This is rather unusual because most single-chain magnets are formed from spins that have axial, rather than planar, anisotropy.
To explore magnetic order and dynamics in this molecule, Watanabe and co-workers used muon spin relaxation measurements performed at the RIKEN-RAL Muon Facility. Muons are charged, subatomic particles with the same spin as a proton. By measuring the evolution of the direction of the muon’s spin in a magnetic material it is possible to determine how the spins in the material are distributed or aligned.
With their measurements, Watanabe and his colleagues could observe the fluctuations of the spins on the FeII ions slow down and finally freeze at around 6 K (-267.15 °C)—a behavior that is similar to what is seen in a broad class of materials known as ‘spin glasses’. Importantly, their measurements have proved that spins with easy-plane anisotropy can order in a single-chain magnet—similar to spins with easy-axis anisotropy.
References
- 1. Kajiwara, T., Watanabe, I., Kaneko, Y., Takaishi, S., Enomoto., M., Kojima, N. & Yamashita, M. Direct observation of the ground-spin alignment of Fe(II)–Fe(III) single chain magnet by muon-spin relaxation. Journal of the American Chemical Society 129, 12360–12361 (2007). doi: 10.1021/ja0741877
