May 2, 2008 Research Highlight Biology
Understanding cancer in mice and men
A new mouse model of human leukemia may provide fresh insights on the genesis of the disease
 Figure 1: Creation of a mouse model of human AML from a severely immunocompromised mouse, which has been injected with human leukemic cells.
Figure 1: Creation of a mouse model of human AML from a severely immunocompromised mouse, which has been injected with human leukemic cells.
Japanese and American researchers have used a mouse model engrafted with human cells to characterize a rare population of cancerous stem cells that gives rise to acute myelogenous leukemia (AML)1.
The cells are hematopoietic stem cells, a subset of bone marrow-derived cells that gives rise to essentially all of the cell types in the blood and immune systems. When isolated from patients with AML, the cells are called leukemic stem (LS) cells, and produce only the immature leukemic cells characteristic of this disease, rather than the range of cells from the blood and immune system produced by healthy hematopoietic stem cells.
“LS cells are the cells that can develop leukemia by self-renewing and generating non-stem leukemic cells. They are not normal hematopoietic stem cells,” says principal investigator Fumihiko Ishikawa. “It is believed that normal stem cells acquire some mutations to become LS cells. But nobody has demonstrated that so far.”
The team, based at the RIKEN Research Center for Allergy and Immunology in Yokohama, developed the mouse model to better study the pathogenic mechanisms leading to the development of human leukemia. The model uses as recipients a strain of mice with a severe immunodeficiency that have been further compromised by a mutation that inactivates a major subset of the immune system’s signaling molecules. These mice cannot recognize human cells as ’non-self’; and by using newborn mice for the engraftment of the human cells, a more robust and longer-lived model is created.
Successes with LS cell engraftment
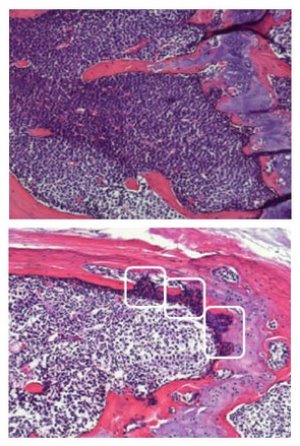 Figure 2: Bone sections derived from AML-engrafted mice before (upper) and after (lower) chemotherapy.
Figure 2: Bone sections derived from AML-engrafted mice before (upper) and after (lower) chemotherapy.
Initially, the researchers demonstrated that human AML cells could be engrafted efficiently into the immunocompromised mice. To do this, human bone marrow cells enriched to contain 80–90% leukemic cells were intravenously injected into mice whose immune systems had been destroyed by sublethal irradiation (Fig. 1). Engraftment of the human cells occurred in nearly 40% of the newborn mice compared with only 13% of adult mice.
Next the researchers showed that the LS cells resided in the subset of the leukemic bone marrow cells expressing the so-called CD34 cell-surface protein but not CD38; as such, the cell-surface protein profile is characteristic of undifferentiated hematopoietic stem cells. When these cells were engrafted into the mouse model, they homed to the bone marrow and were also detectable in the peripheral blood. Injection of other subsets of human leukemic cells, either expressing both CD34 and CD38, or neither of them, did not result in detectable engraftment.
Engraftment was dose dependent, with as few as 1,000 of the LS cells required. Furthermore, these cells were capable of both self-renewal and differentiation into non-stem leukemic cells.
The LS cells could also be serially transferred from mouse to mouse. Serial transplantation over a cumulative period of more than one year successfully demonstrated the long-term self-renewal characteristics of the LS cells.
Specifics on bone-marrow homing
Antibody staining of bone sections showed that the LS cells homed specifically to and engrafted within the micro-environmental niche at the endosteum, a layer of connective tissue on the inner surface of the bone cavity (Fig. 2). This site is where hematopoietic stem cells would normally reside and the process of hematopoiesis, or blood and immune cell differentiation, would take place. The researchers showed that within three days of the LS cells being injected into the mice, the endosteal region, which was essentially empty of cells due to the irradiation required to prepare the mice for engraftment, was lined with LS cells. By 4 months post-engraftment, the bone marrow was packed with AML cells.
The presence of the LS cells suppressed the normal formation of new murine blood cells, a phenomenon also observed in AML patients. The evidence suggests that competition between increasing numbers of LS cells and normal hematopoietic stem cells may be responsible for the eventual suppression of normal hematopoiesis in AML patients.
In addition, the LS cells were relatively resistant to the cytotoxic agent Ara-C, due to the majority of them being in the quiescent (G0) phase of the cell cycle. The researchers believe this explains why AML relapse after chemotherapy is common—non-stem leukemic cells, but not LS cells, are eliminated by cell cycle-dependent cytotoxic agents used to treat the disease. Thus the cell cycle quiescence of the LS cells may protect them from the cytotoxic agents.
Retention of original characteristics
Finally, genetic analysis of the engrafted LS cells showed they retained characteristic gene expression patterns, even after serial transplantation. Analysis of the genes expressed suggests that various genetic targets found in the LS cells may be potential targets for LS cell-specific therapies.
According to Ishikawa, the retention of phenotype, function and gene expression means the model will be a useful tool for the study of the pathogenic mechanisms underlying AML. Using the model, the rare LS cells can be isolated from recipient mice in numbers adequate for large-scale genetic analysis, which may lead to the identification of new therapeutic targets specific to AML. The model may also be useful for testing new therapeutics and treatment modalities for the disease.
“This xenotransplant model will be helpful for developing a cell-bank for human primary AML stem cells and for testing safety and efficacy of various treatment modalities for AML,” Ishikawa notes. “It has already enabled us to identify the major reason and mechanism for AML relapse.”
References
- 1. Ishikawa, F., Yoshida, S., Saito, Y., Hijikata, A., Kitamura, H., Tanaka, S., Nakamura, R., Tanaka, T., Tomiyama, H., Saito, N., et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nature Biotechnology 25, 1315–1321 (2007). doi: 10.1038/nbt1350
About the Researcher
Fumihiko Ishikawa

Fumihiko Ishikawa received an M.D./Ph.D. degree from the Kyushu University School of Medicine for his work on human hematopoiesis using xenotransplantation models under the mentorship of Mine Harada, Takeshi Watanabe and Makio Ogawa. His contributions in the laboratory has been directly related to his experiences in the clinic as a hematologist/oncologist, including in vivo examination of normal human hematopoetic and immune systems, modeling of human hematopoietic malignancies such as AML, ALL and acute T-cell leukemia and the study of human hematopoietic stem cell plasticity. He currently leads the Research Unit for Human Disease Models at the RIKEN Research Center for Allergy and Immunology, where he is continuing his work in hopes of developing new therapeutic approaches to hematopoietic malignancies and other disorders.
