Mar. 14, 2008 Research Highlight Chemistry
Calculating chemistry
Computational models offer a better understanding of how metal-containing reagents participate in chemical reactions
Although nature provides us with a supply of substances that we have adapted for a number of uses, the importance of being able to create ‘designer’ molecules should not be underestimated. Since the birth of the chemical industry in the late 1800s, the benefit to society of laboratory-made pharmaceuticals and materials has been vast.
The evolution of synthetic chemistry has typically occurred through a trial-and-error process—albeit based upon chemists’ observations and intuition. A reaction is optimized by changing the conditions, such as temperature or solvent, or by using different reagents. As pointed out by Masanobu Uchiyama from RIKEN’s Discovery Research Institute in Wako, however, “these processes can be time-consuming and expensive.”
One solution to this problem is to produce theoretical models of how a chemical reaction proceeds so that a deeper understanding of the process can be realized—and the factors that can be changed to improve it. Uchiyama and co-workers have used computational methods to look at how metal-containing compounds react with organic molecules known as enones1.
Enones contain a carbon–carbon double bond that is one bond away from a carbon–oxygen double bond. These compounds undergo reactions (Fig. 1) in which a carbon-based group (R1) can be added in one of two locations. When so-called ‘soft’ metals—such as copper (Cu) or zinc (Zn)—are used, a 1,4-addition reaction puts the R1 group at the ‘4’ position. In contrast, ‘hard’ lithium (Li) reagents cause the R1 group to bond to the ‘2’ position in a 1,2-addition process.
Uchiyama and co-workers investigated the reaction between enones and organozinc reagents by modeling the transition states—the highest energy points along the reaction pathway—for both 1,2- and 1,4-additions. They found that the 1,4-addition proceeds through an ‘open’ transition-state structure that is lower in energy than the more compact one found for 1,2-addition. This result explains the experimentally observed preference for 1,4-addition with organozincates.
Simulations also revealed that while Cu and Zn reagents both lead to 1,4-addition, the reaction mechanism is totally different in each case. Whereas the charge on the Cu atom changes during the reaction, no oxidation/reduction processes are seen in the case of Zn. Thus, an important and long unsolved issue in organic chemistry has been settled by computational chemistry.
“Computational models of these reactions will lead to further modification of organometallic reagents, including zincates,” suggests Uchiyama, “and may lead to improvements in reactivity and selectivity—as well as entirely new reactions.”
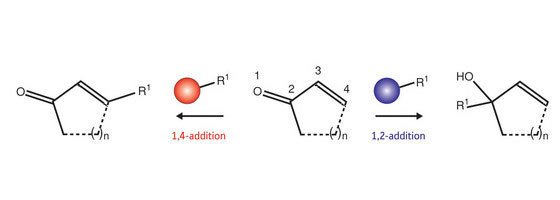 Figure 1: Enones undergo addition reactions with organometallic reagents in which a new carbon chain is added at either the ‘2’ or ‘4’ position. Reagents based on so-called ‘hard’ metals, such as lithium (Li), lead to 1,2-addition (blue), whereas those incorporating ‘soft’ metals, such as copper (Cu) or zinc (Zn), lead to 1,4-addition (red)—also known as ‘conjugate’ addition.
Figure 1: Enones undergo addition reactions with organometallic reagents in which a new carbon chain is added at either the ‘2’ or ‘4’ position. Reagents based on so-called ‘hard’ metals, such as lithium (Li), lead to 1,2-addition (blue), whereas those incorporating ‘soft’ metals, such as copper (Cu) or zinc (Zn), lead to 1,4-addition (red)—also known as ‘conjugate’ addition.
References
- 1. Uchiyama, M., Nakamura, S., Furuyama, T., Nakamura, E. & Morokuma, K. Reaction pathway of conjugate addition of lithium organozincates to s-trans-enones. Journal of the American Chemical Society 129, 13360–13361 (2007). doi: 10.1021/ja070123k
