Mar. 21, 2008 Research Highlight Biology
Immune cells stimulated by calcium levels
Calcium sensors may play a pivotal role in the allergic response
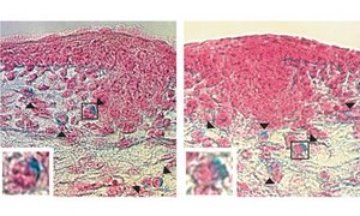 Figure 1: Development of mast cells in normal mouse embryos (left) and embryos lacking the STIM1 protein (right). Arrowheads indicate mast cells stained with alcian blue dye. Insets (bottom left corners) show mast cells in boxes in main images. Reproduced from Ref. 1 © (2008) Nature Publishing Group
Figure 1: Development of mast cells in normal mouse embryos (left) and embryos lacking the STIM1 protein (right). Arrowheads indicate mast cells stained with alcian blue dye. Insets (bottom left corners) show mast cells in boxes in main images. Reproduced from Ref. 1 © (2008) Nature Publishing Group
A team of Japanese researchers is unraveling the role played by calcium ion influx in mast cells and other immune system cells.
Mast cells contain granules consisting of a potent combination of proteins and other bioactive compounds that are released when the cell is stimulated by the binding of immunoglobulin E (IgE) antibodies and an allergy-triggering antigen to specific receptors on the cell surface. This process and the immunological events that accompany it cause the physiological changes characteristic of an allergic response, including swelling, redness and itching.
The team, led by Tomohiro Kurosaki at the RIKEN Research Center for Allergy and Immunology in Yokohama, has focused their work on STIM1: a protein located on the endoplasmic reticulum, a membranous structure within the cell.
Previous studies by Kurosaki and his colleagues have demonstrated that the STIM1 protein physically relocates from the endoplasmic reticulum to regions just under the cell membrane where it is involved in the activation of specific calcium channels known as calcium release-activated channels. This process is known as store-operated calcium influx and is an important mechanism in the activation of mast cells.
Mutation affects calcium influx
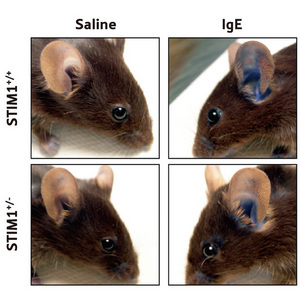 Figure 2: Comparison of in vivo anaphylactic responses in mice with either two functional copies of the STIM1 gene (Stim1+/+) or one functional copy (Stim1+/-). One ear was injected with IgE antibodies against a specific antigen. The other ear was injected with saline as a control. After 16–18 h the antigen was administered intravenously together with a blue dye and the amount of dye leaking out into the ear tissue was measured.
Figure 2: Comparison of in vivo anaphylactic responses in mice with either two functional copies of the STIM1 gene (Stim1+/+) or one functional copy (Stim1+/-). One ear was injected with IgE antibodies against a specific antigen. The other ear was injected with saline as a control. After 16–18 h the antigen was administered intravenously together with a blue dye and the amount of dye leaking out into the ear tissue was measured.
Now, using a genetically engineered mouse strain that does not express the STIM1 protein, the researchers have shown that the calcium influx mediated by stimulation of specific receptors, known as FcεR1, on mast cells was severely impaired1. But the mice have proven difficult to work with: the mutation preventing the expression of STIM1 is lethal with few fetuses surviving beyond birth.
Comparisons between fetal liver-derived mast cells (FLMC) generated from day 15.5 embryos of the mutant mouse strain and a normal mouse embryos exhibited similar morphology and gene expression profiles. The researchers also found that the density of skin mast cells in the embryos was similar. Taken together, these results suggest that mast cell differentiation is not affected by the absence of STIM1 expression.
To circumvent the lethality of the mutation, the researchers used FLMCs from mice lacking STIM1 to examine calcium influx (Fig. 1). Initially the cells were treated with thapsigargin—a compound that inhibits the calcium pumps in the endoplasmic reticulum—and a calcium-chelating agent to deplete calcium stores. When extracellular calcium was added back to the culture, calcium influx in the FLMCs lacking STIM1 was significantly suppressed compared to levels seen in normal FLMCs. A similar result was seen in FLMCs following stimulation of the FcεR1 receptors, suggesting that STIM1-dependent calcium influx was the main mechanism used during FcεR1 signaling.
In the presence of ionomycin, a drug that raises intracellular calcium levels, FLMCs from normal mouse embryos showed consistently high levels of intracellular calcium while the calcium flux in FLMCs lacking STIM1 was much lower.
When the researchers returned the STIM1 gene to the defective cells using a specially modified virus, they found that mobilization of calcium was restored after stimulating the FcεR1 receptors or treating the cells with ionomycin, thereby confirming the importance of STIM1 in bringing in external supplies of calcium.
Impaired immune function
Kurosaki and team have also shown that FLMCs from the mutant mice have impaired immune function. Mast cell granules contain a variety of proteases and other molecules including histamine and β-hexosaminidase, which are responsible for the physiological effects associated with an allergic response. The activation also triggers the cell to produce and secrete other mediators and hormones that promote inflammation.
After stimulation of the FcεR1 receptors of FLMCs lacking STIM1 there was a significant reduction in the release of the mast cell granules, measured by monitoring levels of β-hexosaminidase. When STIM1 expression was restored to the defective cells, the ability of the cells to degranulate was also partially restored. At a molecular level, activation of transcription factors NFAT and NF-κB—which are responsible for regulation of many mast cell functions—was also impaired.
In vivo observations back up in vitro results
Due to the lethality of the mutation, the full effect of deleting STIM1 on the allergic response could not be directly investigated in vivo. But in an experiment using mice with only one functional copy of the gene, the researchers were able to show that the sensitivity of immediate-type allergic responses was diminished, when compared to the response exhibited by mice with two functional copies.
This was demonstrated by sensitizing the ear of the mouse through the injection of IgE antibodies against a specific antigen into the outer part of the ear, a process called passive cutaneous anaphylaxis. Sixteen hours after sensitization, the antigen was injected intravenously, in combination with a blue dye. Degranulation of mast cells causes increased permeability of blood vessels in the sensitized site, and the dye leaks into surrounding tissues where it can be biopsied and quantified.
In sensitized mice with only one functional copy of the STIM1 gene, the amount of dye entering the tissues was significantly lower than in mice with two copies (Fig. 2), suggesting that the protein is required for antigen-induced, mast cell-mediated anaphylactic responses in vivo.
While there are still questions to be answered about precisely how STIM1 mediates calcium influx, there is no doubt that the protein plays an important role in regulating intracellular calcium levels in mast cells.
“STIM1 may represent a new therapeutic target for allergic diseases,” team-member Yoshihiro Baba says.
The next step, says Baba, will be to use a conditional system that only blocks expression of STIM1 in specific cell types, rather than the whole animal.
References
- 1. Baba, Y., Nishida, K.,Fujii, Y., Hirano, T., Hikida, M. & Kurosaki, T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nature Immunology 9, 81–88 (2008). doi: 10.1038/ni1546
About the Researcher
Tomohiro Kurosaki

Tomohiro Kurosaki was born in Okayama, Japan, in 1955. He received his M.D. in 1980 from Okayama University Medical School and his Ph.D. in 1987 from Kyoto University. After he obtained his Ph.D., he did his postdoctoral fellowship at the Sloan-Kettering Institute in New York. He then joined Lederle Laboratories till 1996 as a senior research scientist, while holding the position of adjunct assistant professor in Yale University in the United States. After returning to Japan, he directed and taught at the Institute for Liver Research at Kansai Medical University. He joined RIKEN in 2001 and has been director of his own research group. His laboratory focuses on understanding the molecular composition of preBCR/BCR signaling complexes and the mechanisms of signaling pathway crosstalk that lead to crucial cell fate decisions during B lymphocyte differentiation. He has also applied insights gained from the studies of B cells to another important immune effecter cell, the mast cell.
