Apr. 11, 2008 Research Highlight Physics / Astronomy
Spins in nickel stand together
Theorists extend a simple model to explain ferromagnetism in transition metals
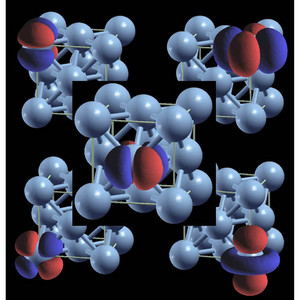 Figure 1: The face-centered cubic (FCC) lattice structure of atoms (blue spheres) is a common structure of pure metals, such as nickel. Each of the five images shows one of the possible multiple orbitals (red and blue lobes) for the 3d transition metal ions (found in the fourth row of the periodic table). For clarity, each orbital is drawn on a different FCC lattice.
Figure 1: The face-centered cubic (FCC) lattice structure of atoms (blue spheres) is a common structure of pure metals, such as nickel. Each of the five images shows one of the possible multiple orbitals (red and blue lobes) for the 3d transition metal ions (found in the fourth row of the periodic table). For clarity, each orbital is drawn on a different FCC lattice.
Physicists have solved various exotic problems yet the familiar phenomenon of magnetism in metals, such as iron and nickel, has remained difficult to explain.
Now Ryotaro Arita of the RIKEN Discovery Research Institute at Wako and his colleagues Shiro Sakai and Hideo Aoki at the University of Tokyo have shown the importance of two elements in predicting magnetism in transition metals1—lattice structure and multiple electron orbitals (or energy levels), on the magnetic ions. “This work will have an impact on a long-standing condensed matter physics problem,” explains Arita. “Now we know how electrons can conduct current with their spins aligned at the same time.”
The group based their calculations on an extension of the Hubbard model, one of the simplest solid state physics models that takes into account interactions between electrons in a material. Previous work showed that the Hubbard model can explain certain magnetic states, but not ferromagnetism—the state in which all spins are aligned—in real metals.
In its simplest form, the Hubbard model describes a lattice of atoms with one orbital per atom. If only one electron occupies the orbital on an atom, there is no cost in energy, but if two electrons occupy the same orbital there is a large energy cost. Moreover, the spins of two electrons on the same orbital cannot point in the same direction because of the so-called Pauli exclusion principle. The final arrangement of the electrons—and their spins—depends on the balance between the ‘repulsive’ energy between electrons on the same site and the kinetic energy that electrons gain by moving around the lattice.
The Hubbard model is attractive for theorists because it provides a clear microscopic picture of magnetism. Arita and colleagues therefore built on the ‘simple’ Hubbard model, to see if it could predict ferromagnetism in transition metals. In their model, each atomic site contains multiple electron orbitals (Fig. 1). Since electrons on the same atom can occupy distinct orbitals, their spins can point in the same direction without violating the Pauli exclusion principle. The group shows this effect is important for obtaining ferromagnetism in a metal.
Arita and colleagues also considered the role of lattice structure. They showed their model predicts ferromagnetism in transition metals with a ‘face centered cubic’ structure (Fig. 1) but not a ‘simple cubic’ structure.
The team’s model is successful at explaining ferromagnetism in nickel and may ultimately be applied to more complex materials.
References
- 1. Sakai, S., Arita, R. & Aoki, H. Itinerant ferromagnetism in the multiorbital Hubbard model: A dynamical mean-field study. Physical Review Letters 99, 216402 (2007). doi: 10.1103/PhysRevLett.99.216402
