Apr. 18, 2008 Research Highlight Chemistry
Porous networks in an instant
The scope for materials with internal cavities widens—with a fast synthetic route and accurate structural characterization
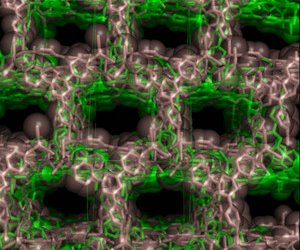 Figure 1: The crystal structure of the network synthesized under kinetic control. Reproduced with permission from Ref 1. © 2008 Wiley-VCH Verlag GmbH & Co. KGaA
Figure 1: The crystal structure of the network synthesized under kinetic control. Reproduced with permission from Ref 1. © 2008 Wiley-VCH Verlag GmbH & Co. KGaA
Researchers at the RIKEN Advanced Science Institute, the formerly Discovery Research Institute in Wako, and the University of Tokyo report in the international edition of Angewandte Chemie1 that they have synthesized a kinetically controlled porous three-dimensional network, known as a coordination network, and determined its structure using the SPring-8 synchrotron in Harima. The finding should extend the range of physical properties and applications of these porous materials.
Three-dimensional porous frameworks are comprised of an ordered assembly of metal ions and ligands and have many applications in science. Synthesizing porous coordination networks is difficult, however, as non-porous networks often result.
The networks can be produced under thermodynamic or kinetic control, which result in products of varying stability. The so-called thermodynamic product is the most stable of multiple products formed in a reaction, whereas the kinetic product will form the fastest but will not be the most stable.
By controlling the rate of complexation of the starting materials, the researchers produced two different types of porous network. Using a slow crystallization process under thermodynamic control, taking about one week, they produced a network that has very large one-dimensional channels formed from weak interactions between the ligands. However, at a faster rate under kinetic control, taking less than 30 seconds, they produced porous network with smaller holes.
As the latter product is formed almost instantaneously, there is insufficient time for a single crystal to grow, which is needed to determine the structure of these frameworks using conventional x-ray diffraction studies. Instead, the team isolated a uniform microcrystalline powder but found that its structure could be determined by synchrotron powder x-ray diffraction (Fig. 1). According to the researchers, this is the first time a kinetically controlled network with large cavities has been successfully characterized using this technique. Determining the structure of these frameworks is crucial for advancing the applications of these networks.
The researchers believe that this instant synthesis, coupled with the ability to characterize the microcrystalline product, could lead to advances in the area of coordination network chemistry. For example, as the pore size can be tuned by changing the ligands, it may be possible to make porous materials that achieve a larger amount of gas adsorption.
“The results suggest that more unexplored kinetic networks can be prepared and characterized by synchrotron powder x-ray diffraction,” says team-member Masaki Kawano from the University of Tokyo.
References
- 1. Kawano, M., Haneda, T., Hashizume, D., Izumi, F. & Fujita, M. A selective instant synthesis of a coordination network and its ab initio powder structure determination. Angewandte Chemie International Edition 120, 1289–1291, (2008). doi: 10.1002/anie.200704809
