May 23, 2008 Research Highlight Biology
Transfusable blood from a stem cell
A new technique for growing blood cells in vitro may reduce the need for blood donors
Researchers at the RIKEN BioResource Center in Tsukuba have established several cell lines that produce functional red blood cells (RBCs) from mouse embryonic stem cells1. The technique may pave the way for production of human donor red blood cells in vitro, lessening the need for blood donation.
According to team leader Yukio Nakamura, both embryonic and adult stem cells have been used in the past to establish red blood cell-like cell lines. But this is the first time, he says, that researchers have shown the feasibility of using stem-cell derived cell lines to efficiently produce red blood cells.
“To date, the use of hematopoietic cells produced in vitro has not proved practical for routine therapeutic applications,” Nakamura says.
A step-wise approach
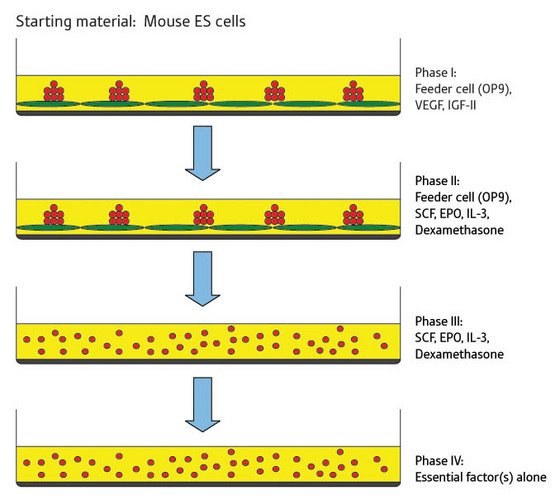 Figure 1: The four stages of cell culture required to generate red blood cell-producing cell lines from embryonic stem cells in mice. The green cells are feeder cells and the red cells are the embryonic stem cell-derived cell line.
Figure 1: The four stages of cell culture required to generate red blood cell-producing cell lines from embryonic stem cells in mice. The green cells are feeder cells and the red cells are the embryonic stem cell-derived cell line.
The researchers initially induced hematopoiesis—that is, differentiation from the stem cell into more mature blood and immune system cells—in mouse embryonic stem cells by culturing the stem cells on a layer of so-called feeder cells in the presence of several specific growth factors as well as the synthetic steroid drug dexamethasone.
Four consecutive phases of cell culture were used to induce differentiation (Fig. 1). Initially the cells were cultured on the feeder cells in the presence of vascular endothelial growth factor (VEGF) and insulin-like growth factor II (IGF-II). After four days the free-floating (or detached) cells were transferred to a new layer of feeder cells and cultured in the presence of a new set of growth factors including stem cell factor (SCF), erythropoietin (EPO) and interleukin-3 (IL-3). At this stage dexamethasone was also added to the culture.
This second phase of cell culture was maintained over a period of 60 days or more, at which point the researchers started to test the detached cells in the culture for the ability to grow in the absence of feeder cells. Once the cells were growing well in the absence of feeder cells, each cell line was tested to establish which cell growth factors were essential for continued growth.
The team reports they established a total of five cell lines by this method. Three cell lines exhibited characteristics of immature red blood cell, or progenitor erythroid, lineages. The other two lines exhibited the characteristics of mast cells, a type of immune system cell.
Cells function as expected
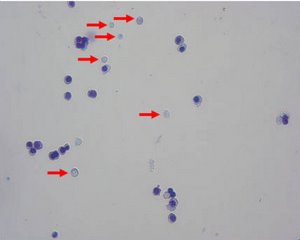 Figure 2: The erythroid cell line could be induced to further differentiate, generating mature red blood cells lacking nuclei (marked by arrows).
Figure 2: The erythroid cell line could be induced to further differentiate, generating mature red blood cells lacking nuclei (marked by arrows).
Analysis of the three erythroid cell lines demonstrated that the cells expressed genes specific for erythroid cells. Furthermore, the cells expressed α and β globin chains, which are only expressed by adult erythroid cells. But each cell line appeared to be at a slightly different stage of differentiation, demonstrating that the erythroid progenitor cells could be immortalized at different stages.
By altering the growth conditions, the team could induce each erythroid-like cell line to further differentiate in vitro producing more mature erythroid cells, including red blood cells (Fig. 2).
Next, the researchers examined the ability of the erythroid cell lines to produce red blood cells in vivo in mice suffering from acute anemia. A subline of one of the erythroid cell lines was modified to express a green fluorescent protein marker and these cells were injected into the anemic mice. A transient proliferation of the marker-expressing cells was observed, but as differentiation of the cells occurred they lost the ability to express the marker making it more difficult to track the transplanted cells. However injection of the erythroid cell lines ameliorated the anemia, suggesting that differentiation of the injected cells occurred in vivo to produce red blood cells similar to those produced naturally by the host mouse.
Further investigation showed that the cell-line derived cells differentiated in vivo into much more mature lineages than seen in vitro.
Tumor-free transplants
The researchers also evaluated the potential for the cell lines to form tumors in vivo as some stem cell lines have been associated with tumorigenicity. The cell lines generated by the RIKEN group were shown to include some cells containing abnormal chromosomes, a common occurrence in long-term cell cultures. Mature red blood cells lack a nucleus, and hence lack DNA, so carry little risk of tumorigenesis, but the less mature cells produced by the cell line do contain DNA.
The team made two observations that imply that these cells are not tumorigenic. Cells expressing the fluorescent marker disappeared about 3 months after transplantation into the mice. And, no tumors were detected in other mice observed for a period of up to 6 months following transplantation with the cell lines. However, the researchers suggest that human cell lines generated for clinical use might need to be periodically recloned to maintain their genotype.
Future transfusions
Nakamura says it should be possible to use the same method to produce human erythroid cell lines capable of producing red blood cells, platelets and other useful blood cells, which could reduce dependence on volunteer blood donations.
“There is little doubt that red blood cells, platelets, and neutrophils produced in vitro would be candidate materials to replace cells donated from such a large group of anonymous individuals,” he says.
References
- 1. Hiroyama, T., Miharada, K., Sudo, K., Danjo, I., Aoki, N. & Nakamura, Y. Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells. PLoS ONE 3(2), e1544 (2008). doi: 10.1371/journal.pone.0001544
About the Researcher
Yukio Nakamura

Yukio Nakamura was born in Nagano, Japan, in 1961. He graduated from the Niigata University (School of Medicine) in 1986, obtained his license as a medical doctor in 1986, and obtained his PhD in 1996 from Tsukuba University. After four years working as a medical doctor, he moved to RIKEN (Tsukuba Life Science Center) in 1990, where he started his career as a basic hematologist. He moved to Tsukuba University as an assistant professor in 1994 and then moved to the Walter and Eliza Hall Institute (Melbourne) as a visiting scientist. He was promoted to principal investigator of RIKEN in 2002 and then to division head of RIKEN in 2003. His research focuses on the establishment of useful cell lines and the in vitro production of blood cells that can be used in the clinic.
